Dmitriev Lab: Copper, Iron, Platinum and the Proteins that Handle them
Information on our research, people and publications
Research Projects
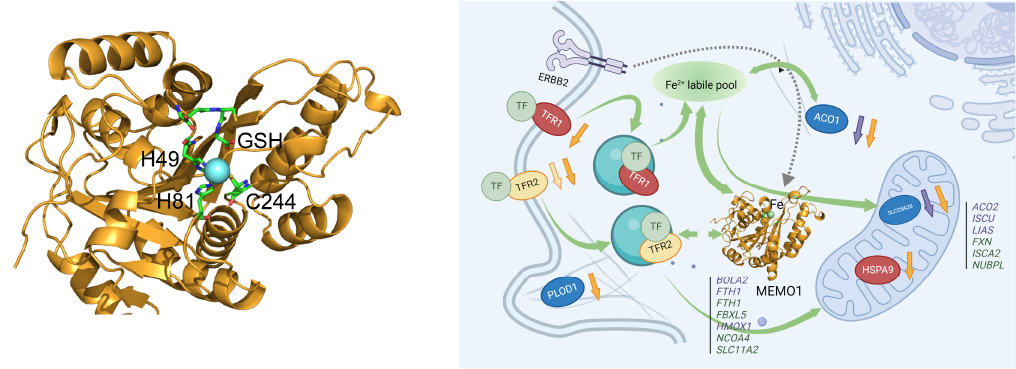
ATP7B (Wilson disease protein) is a human copper transporter powered by ATP hydrolysis. ATP7B delivers copper to some proteins that use it as a cofactor, and also removes excess copper from the cell in a complex process that involves copper translocation across the lipid membrane and fusion of membrane vesicles with the plasma membrane. Activity of ATP7B is regulated by copper binding to six small and highly mobile cytosolic metal-binding domains, which are connected by flexible linkers. We use protein NMR spectroscopy to study how dynamics and interactions of the metal-binding domains signal activation of copper transport and direct ATP7B localization in the cell.
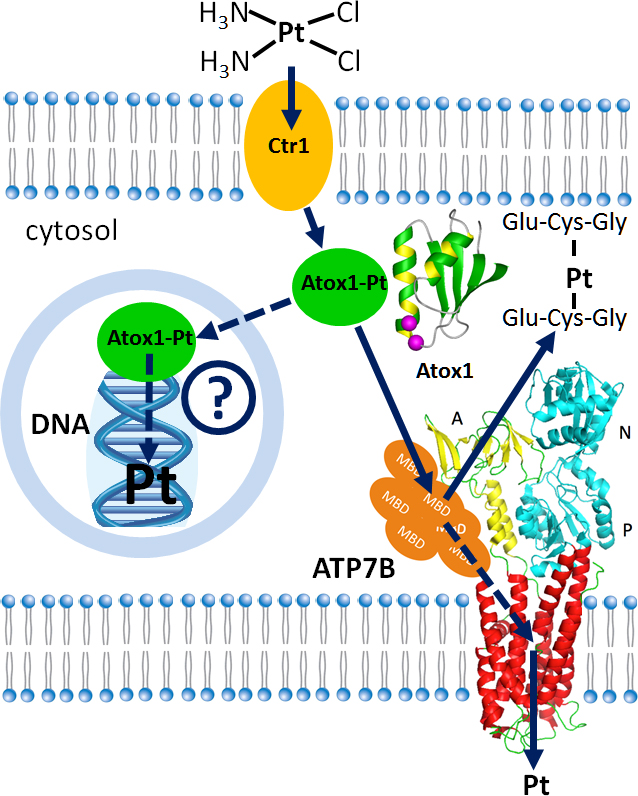
Cisplatin, carboplatin and oxaliplatin are platinum derivatives that are widely used to treat several common types of cancer. Cancer cells often develop resistance to platinum drugs and that undermines effectiveness of chemotherapy. We are investigating the role that copper transport proteins Atox1 and ATP7B play in cancer resistance to cisplatin and similar drugs. Our work has uncovered remarkable parallels between the pathways of copper and platinum transport in the cell. Manipulating these pathways in cancer cells may increase effectiveness, and reduce toxicity of platinum anticancer drugs.