The latest
Metabolic Effects of the Cancer Metastasis Modulator MEMO1
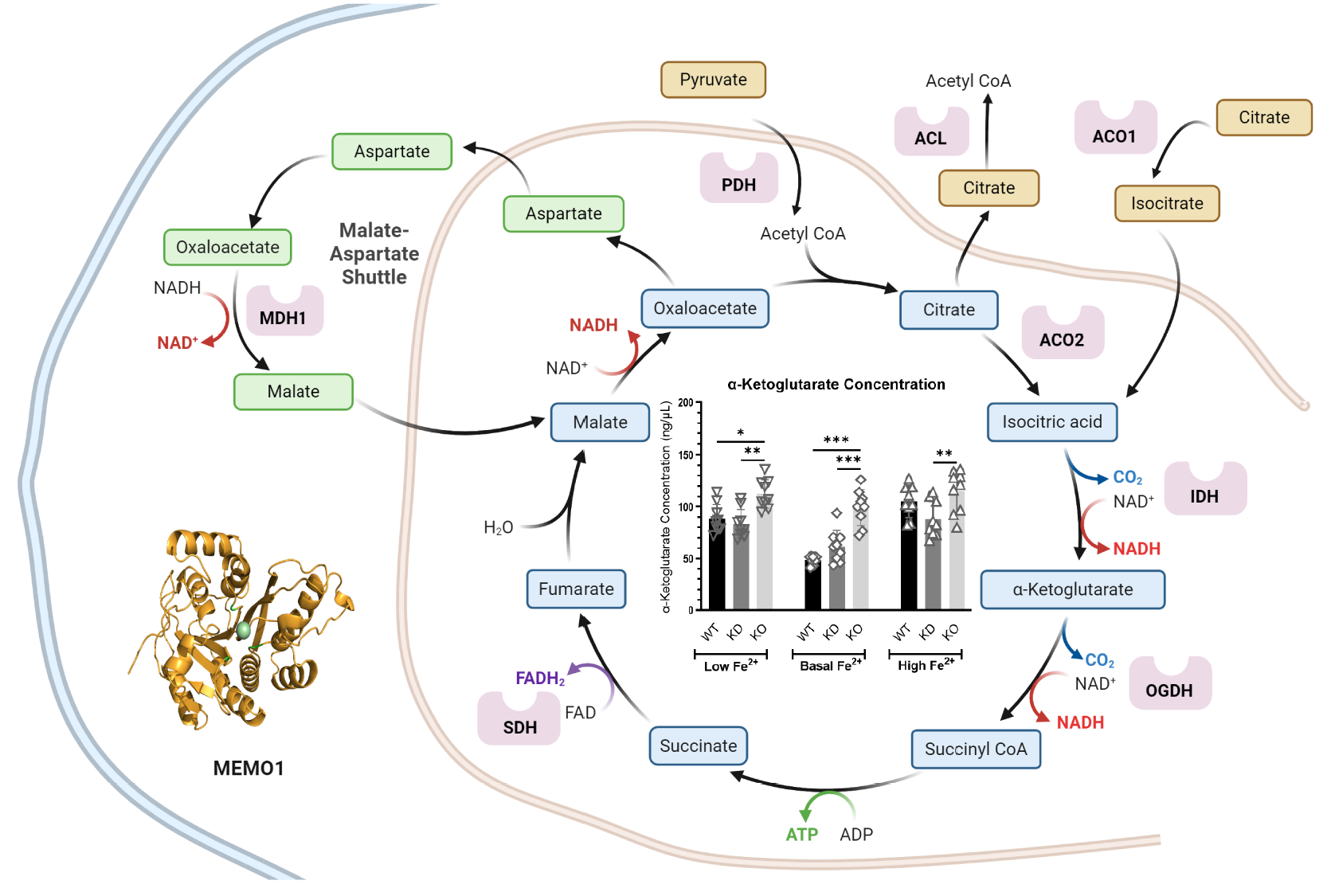
Ghanbarian, M., Dolgova, N., Vizeacoumar, F.S., Vizeacoumar, F.J., Michel, D., El-Aneed, A., Dmitriev,
O.Y. (2025) Metabolites, 15: 277 (1-17)
MEMO1 binds iron and modulates iron homeostasis in cancer cells
Dolgova, N., Uhlemann, E. E., Boniecki, M. T., Vizeacoumar, F. S., Ara, A., Nouri, P., Ralle, M., Tonelli, M., Abbas, S. A., Patry, J., Elhasasna, H., Freywald, A., Vizeacoumar, F. J., and Dmitriev, O.Y. (2024) Elife, 13:e86354
Structure and mechanism of the human copper transporting ATPases: Fitting the pieces into a moving puzzle
Dmitriev, O.Y. and Patry, J. (2024) Biochim. Biophys. Acta Biomembr.
At Sixes and Sevens: a Cryptic Domain in the Metal Binding Chain of the Human Copper Transporter ATP7A
Uhlemann, E.E., Lee, W., Tonelli, M. and Dmitriev, O.Y. (2021) Biophys. J.
Nanobodies Against the Metal Binding Domains of ATP7B as Tools to Study Copper Transport in the Cell
Uhlemann, E.E., Yu, C.H., Patry, J., Dolgova, N., Lutsenko, S., Muyldermans, S. and Dmitriev, O.Y. (2020) Metallomics, 12: 1941-1950
Engineered Protein Model of the ATP synthase H+- Channel Shows No Salt Bridge at the Rotor-Stator Interface.
Pierson, H.E., Kaler, M., O’Grady, C., Uhlemann, E.E., and Dmitriev, O.Y. (2018) Sci. Rep. 8: 11361.
The Structure of Metal Binding Domain 1 of the Copper Transporter ATP7B Reveals Mechanism of a Singular Wilson Disease Mutation.
The metal chaperone Atox1 regulates the activity of the human copper transporter ATP7B by modulating domain dynamics.
Yu, C.H., Yang, N., Bothe, J., Tonelli, M., Nokhrin, S., Braiterman, L., Dolgova, N.V., Lutsenko, S. and Dmitriev, O.Y. (2017) J. Biol. Chem., 292: 18169-18177.
Binding of Copper and Cisplatin to Atox1 is Mediated by Glutathione through the Formation of Metal-Sulfur Clusters.
Dolgova, N.V., Yu. C., Cvitkovic, J.P., Hodak, M., Nienaber, K., Summers, K.L., Cotelesage, J., Bernholc, J., Kaminski, G.A., Pickering, I.J., George, G.N., and Dmitriev, O.Y. (2017), Biochemistry, 56: 3129–3141
Dynamics of the metal binding domains and regulation of the human copper transporters ATP7B and ATP7A.
Yu, C.H., Dolgova, N.V., and Dmitriev, O.Y. (2017) IUBMB Life, 69: 226-235
Nanobodies as Probes for Protein Dynamics in Vitro and in Cells
Dmitriev, O.Y., Lutsenko, S., and Muyldermans, S. (2016) J. Biol. Chem., 291: 3767-3775
Interactions Between Metal-Binding Domains Modulate Intracellular Targeting of Cu(I)-ATPase ATP7B, as Revealed by Nanobody Binding
Huang, Y., Nokhrin, S., Hassanzadeh-Ghassabeh, G., Yu, C., Yang,H., Barry, A.N., Tonelli, M.. Markley, J.L., Muyldermans, S., Dmitriev, O.Y., Lutsenko, S. (2014) J. Biol. Chem., 289:32682-93
The use of nanopore analysis for discovering drugs which bind to α-synuclein for treatment of Parkinson's disease
Tavassoly, O., Kakish, J., Nokhrin, S., Dmitriev, O.Y. and Lee, J.S. (2014) Eur. J. Med. Chem. 88:42-54
Cu(II) and dopamine bind to α-synuclein and cause large conformational changes.
Tavassoly, O., Nokhrin, S., Dmitriev, O.Y. and Lee, J.S. (2014) FEBS J., 281(12):2738-53
Copper chaperone Atox1 interacts with the metal-binding domain of Wilson disease protein in cisplatin detoxification.
Dolgova, N.V., Nokhrin, S., Yu, C., George, G.N., and Dmitriev, O.Y. (2013) Biochem. J. 454:147-56
One-Step Amino Acid Selective Isotope Labeling of Proteins in Prototrophic E. coli Strains,
O’Grady, C., Rempel, B.L., Sokaribo, A. and Dmitriev, O.Y. (2012) Anal. Biochem. 426, 126-128
Molecular Events Initiating Exit of a Copper-Transporting ATPase ATP7B from the Trans-Golgi Network
Hasan, N.M., Gupta, A., Polishchuk, E., Yu, C.H., Polishchuk, Dmitriev, O.Y. and Lutsenko, S. (2012) J. Biol.Chem. 287, 36041–36050
Cell-free synthesis of membrane subunits of ATP synthase in phospholipid bicelles: NMR shows subunit a fold similar to the protein in the cell membrane.
Uhlemann, E.E., Pierson, H.E, Fillingame, R.H. and Dmitriev, O.Y. Protein Sci. 21, 279-288
Interaction With Monomeric Subunit c Drives Insertion of ATP Synthase Subunit a into the Membrane and Primes a-c Complex Formation.
Pierson, H.E., Uhlemann, E.E. and Dmitriev, O.Y. (2011) J. Biol.Chem. 286, 38583-38591
Difference in Stability of the N-Domain Underlies Distinct Intracellular Properties of the E1064A and H1069Q Mutants of Cu-transporting ATPase ATP7B.
Dmitriev, O.Y., Bhattacharjee, A., Nokhrin, S., Uhlemann, E.E., and Lutsenko, S. (2011) J. Biol.Chem. 286, 16355-16362
Disease Mutation or Polymorphism? Cellular Copper Levels Determine the Phenotype of the Arg875 Variant of ATP7B/Wilson Disease
Gupta, A., Bhattacharjee, A., Dmitriev, O., Nokhrin, S., Braiterman, L., Hubbard, A., Lutsenko, S. (2011) Proc.Natl.Acad.Sci. USA, 108, 5390-5395
Mechanism Of Tumor Resistance To Cisplatin Mediated by the Copper Transporter ATP7B
Dmitriev, O.Y. (2011) Biochem. Cell Biol. 89, 138-147
Crystallization and Preliminary X-ray studies of the N-domain of the Wilson Disease Associated Protein
Liu, L., O'Grady, C., Dalrymple, S.A., Prasad, L., Dmitriev, O. Y., and Delbaere, L.T. (2009) Acta Crystallographica F 65:621-624
The soluble metal-binding domain of copper transporter ATP7B binds and detoxifies cisplatin.
Dolgova, N.V., Olson, D., Lutsenko, S., and Dmitriev, O. Y. (2009) Biochem. J, 419, 51-56
Interaction of Transmembrane Helices in ATP Synthase Subunit a in Solution as Revealed by Spin-Label Difference NMR
Dmitriev, O. Y., Freedman, K. H., Hermolin, J. and Fillingame, R.H. (2008) Biochim. Biophys. Acta (Bioenergetics), 1777: 227-237
Function and Regulation of Human Copper-Transporting ATPases
Lutsenko, S., Barnes, N.L., Bartee, M.Y., Dmitriev, O.Y. (2007) Physiol. Revs., 87: 1011-1046
The Rigid Connecting Loop Stabilizes Hairpin Folding of the Two Helices of the ATP Synthase Subunit c.
Dmitriev, O. and Fillingame, R.H. (2007) Protein Science 16: 2118-2122
Solution Structure of the N-domain of Wilson Disease Protein: Unique Nucleotide-Binding Environment and Effects of Disease Mutations
Dmitriev, O., Tsivkovskii, R., Abildgaard, F., Morgan, C.T., Markley, J.L. and Lutsenko, S. (2006) Proc. Natl. Acad. Sci. USA 103: 5302-5307
NMR assignment of the Wilson Disease Associated Protein N-Domain
Dmitriev, O., Tsivkovskii, R., Abildgaard, F., and Lutsenko, S. (2006) J. Biomol. NMR, 36, Suppl. 1:61
