We are interested in the structure, mechanism and evolution of glycosaminoglycan (GAG) processing enzymes. We have determined the 3-D structures and characterized the reaction mechanisms of several GAG lyases, concentrating on enzymes that degrade chondroitin and dermatan sulfates, heparan sulfate and heparin. Structural insights from this work have established new evolutionary relationships between polysaccharide lyases (PL) not evident from sequences alone, and also illustrate convergent evolution of their active sites.
To further define evolutionary connections between polysaccharide lyases we are expanding our structural and functional studies to include the two remaining structurally uncharacterized PL families. We will also address structural and mechanistic questions related to the synthesis of GAGs and their modifications. GAG synthases have to decide at each extension step which of the two sugar components has to be added next, and the molecular mechanism of this process is still unknown. Furthermore, the post-synthesis modifications of GAGs are non-randomly distributed along the GAG chains but cluster together, govern by an unknown mechanism, and this clustering is essential for a variety of GAG functions.
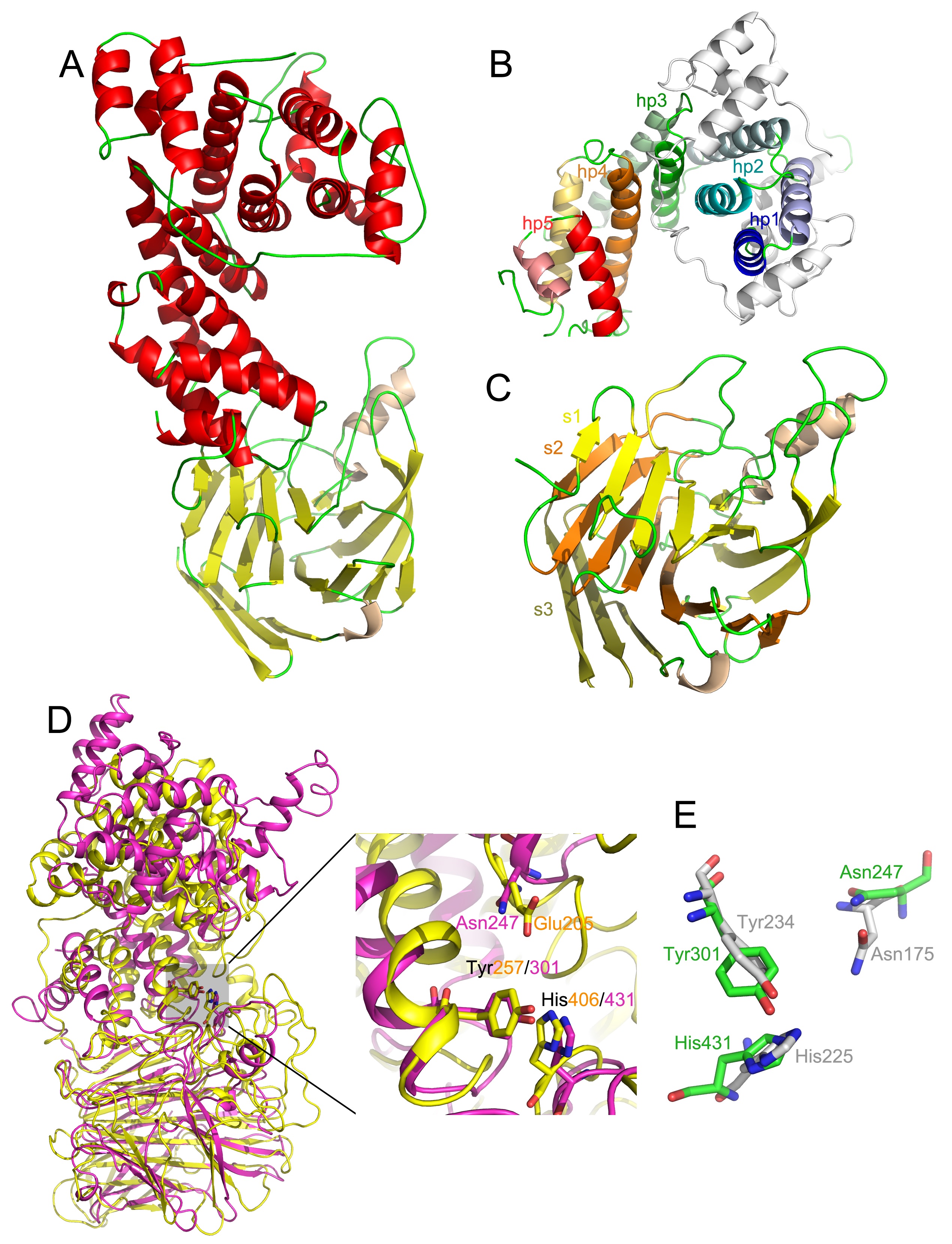
Another example of a polysaccharide lyase is heparinase III from Bacteroides thetaiotaomicron. We have recently characterized this enzyme structurally and functionally.