CREB3L1
A metastasis suppressor protein in breast cancer
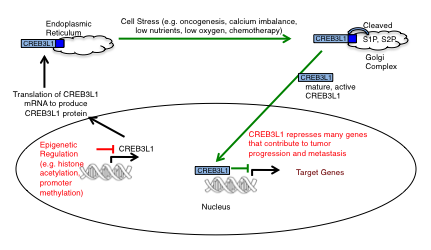
We are characterizing the metastasis suppressor protein, CREB3L1, activated during the stressful conditions (low nutrients and low oxygen) present in tumors. This transcription factor represses the expression of genes involved in cell growth, cell survival, migration and invasion, and is lost in highly metastatic breast cancer cells (Mellor et al., 2013; Ward et al., 2016). In breast cancer cell lines, CREB3L1 is typically expressed in non-tumorigenic cells, as well as tumorigenic but poorly metastatic cells, but its expression is reduced in highly metastatic cells (Mellor et al., 2013). CREB3L1-deficient breast cancer cells show enhanced cell migration and invasion properties, and form colonies in soft agar. Re-expression of CREB3L1 causes decreased cell migration and invasion, and also significantly decreases the ability of cells to form colonies in soft agar. Similarly, re-expression of CREB3L1 in a rat model of breast cancer reduces the growth of the primary tumor and completely blocks metastases, in part as a result of poor blood vessel development in the primary tumors. These results show that CREB3L1 expression represses the tumorigenic and migratory properties of breast cancer cells.
Our recent publication analyzed CREB3L1 expression (mRNA and protein) in >200 breast tumor specimens and extended the mRNA analysis using the TCGA database to an additional >400 samples (Ward et al., 2016). We discovered that CREB3L1 expression is up regulated in low- and medium-grade tumors, as compared to normal breast cells. In contrast, high-grade (8 and 9) breast tumors had reduced CREB3L1 expression (p = 0.001). Our results suggest that CREB3L1 expression is initially up regulated in response to the stressful conditions that exist within the tumor environment, as observed for other stress response proteins. In contrast to other stress response proteins, loss of CREB3L1 expression is prevalent in high-grade tumors and may be required to avoid apoptosis under prolonged stress conditions. This would allow the de-repression of genes necessary for cancer progression and metastasis. Overall, 30% of breast cancers have low levels of CREB3L1 and these patients have a poor prognosis as compared to patients with breast tumors expressing CREB3L1. We are focused on identifying targets to block the growth of, or selectively kill, CREB3L1-deficient breast cancer cells that are typically the most metastatic.
 |
 |
|
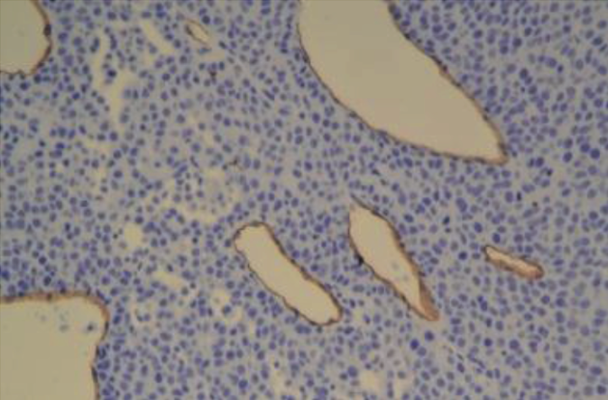
Tumor vessels - no CREB3L1 |
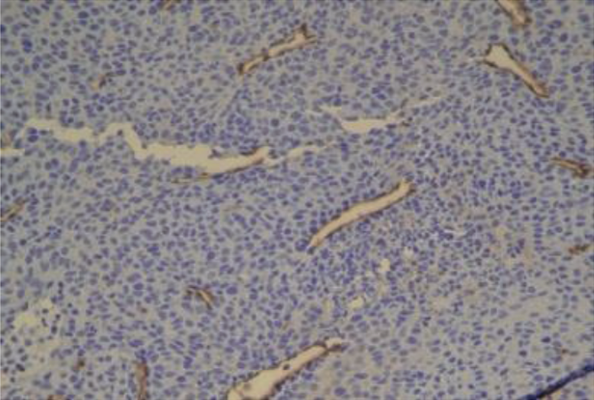
Tumor vessels + CREB3L1 |
