Lead Halide Perovskites
Since their initial development in 2009, perovskite solar cells have emerged as one of the most promising new photovoltaic technologies. Power conversion efficiencies have increased from less than 4% in 2009 to more than 22% today, making these devices increasingly competitive with more traditional multicrystalline silicon and cadmium telluride technology. Our research efforts in this area span a diverse range of topics, and our current work is focused on improving the lifetime and performance of perovskite solar cells, investigating perovskite degradation mechanisms using in situ spectroscopy and in situ diffraction techniques, and the synthesis of novel hole- and electron-transport materials.
Device Engineering
The original designs of perovskite solar cells were derived from those of DSSCs, and utilized mesoporous TiO2 electron-transport layers. Our earliest work in this area demonstrated that a compact layer of ZnO nanoparticles could be used to replace the mesoporous TiO2, and that the resulting planar heterojunction devices worked extremely well [Nature Photon., 2014]. Subsequent work investigated the impact of perovskite layer thickness on device efficiency, and revealed that the high efficiency of these devices results from a good match between the light absorption depth and the charge carrier diffusion length [J. Mater. Chem. A, 2014].
Although we have continued to develop ways of improving device efficiency, much of our recent work has focused on the single greatest challenge facing this technology - short device lifetimes. Recent work has highlighted the instability of most lead halide perovskites when faced with environmental stresses like moisture, oxygen, light, and heat. Whether it is through careful modulation of the perovskite composition [ACS Appl. Energy Mater., 2018] or through the development of nanostructured hole-transport layers [Mater. Chem. Front., 2018], we continue to find innovative ways of producing more stable materials and longer-lasting devices.
Elucidating Degradation Mechanisms

In order to design devices with longer lifetimes, we first need to understand how and why these lead halide perovskites decompose. We have developed a suite of humidity-controlled sample holders and environmental test chambers that allow us to probe both thin films and devices using in situ UV/vis spectroscopy and grazing-incidence wide angle X-ray scattering (GIWAXS). These unique capabilities have allowed us to answer fundamental questions about the reactivity of these materials.
Our early work demonstrated the importance of hydrate phases in the decomposition pathway [ACS Nano, 2015]; we continue to explore the influence of interfacial layers, perovskite composition, and electrode corrosion on the degradation mechanisms of perovskite solar cells.
Selected Publications on Lead Halide Perovskites
- K. Poorkazem, and T. L. Kelly* Compositional Engineering to Improve the Stability of Lead Halide Perovskites: A Comparative Study of Cationic and Anionic Dopants. ACS Applied Energy Materials. 2018, 1, 181-190. [DOI: 10.1021/acsaem.7b00065]
- S. Kundu, and T. L. Kelly* Improving the moisture stability of perovskite solar cells by using PMMA/P3HT based hole-transport layers. Materials Chemistry Frontiers. 2018, 2, 81-89. [DOI: 10.1039/C7QM00396J]
- J. Yang, and T. L. Kelly* Decomposition and Cell Failure Mechanisms in Lead Halide Perovskite Solar Cells. Inorganic Chemistry. 2017, 56, 92-101. [DOI: 10.1021/acs.inorgchem.6b01307]
- J. Yang, B. D. Siempelkamp, D. Liu, and T. L. Kelly* Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in situ Techniques. ACS Nano. 2015, 9, 1955-1963. [DOI: 10.1021/nn506864k]
- D. Liu, M. K. Gangishetty, and T. L. Kelly* Effect of CH3NH3PbI3 Thickness on Device Efficiency in Planar Heterojunction Perovskite Solar Cells. Journal of Materials Chemistry A. 2014, 2, 19873-19881. [DOI: 10.1039/c4ta02637c]
- D. Liu, and T. L. Kelly* Perovskite Solar Cells with a Planar Heterojunction Structure Prepared using Room-Temperature Solution Processing Techniques. Nature Photonics. 2014, 8, 133-138. [DOI: 10.1038/NPHOTON.2013.342]
Organic Electronics
Our research group is also heavily involved in the development of organic electronics, in particular organic photovoltaic (OPV) technology. OPVs have improved tremendously over the past 15 years, and they are now in the early stages of commercialization. This success is in part due to the unique advantages of OPVs: the cells can be prepared using highly-scalable solution-based fabrication methods; they can be prepared in a wide variety of attractive colors and with unique form factors; and they can be made fully transparent, facilitating their integration into building design. Yet despite this success, OPV technology lags behind other areas of organic electronic (e.g., OLEDs and OFETs). Our research targets the lingering issues with this technology through the synthesis of new materials and the development of highly scalable processing methodologies.
Synthesis of New Materials
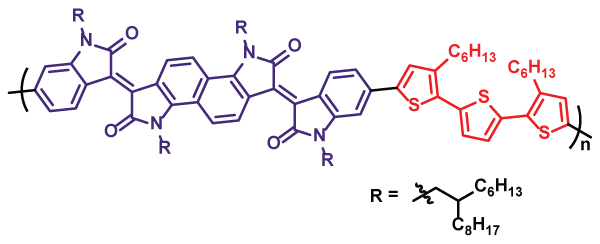
One important part of our research program is the synthesis of new organic optoelectronic materials. Our past work has produced both polymeric and molecular systems, with an emphasis on low bandgap materials inspired by the isoindigo unit. A representative example is the donor-acceptor copolymer shown here, which is based on "bisisoindigo" - a ring-fused dimer of isoindigo. In the solid state, it absorbs out to nearly 1100 nm, giving it an optical bandgap comparable to that of silicon! Our current work emphasizes the synthesis of new electron-transport materials and non-fullerene acceptors.
Scalable Processing Methods
Perhaps the greatest advantage of OPVs is their potential to be rapidly manufactured at scale using roll-to-roll processing methods. Yet the vast majority of research in the OPV literature has focused on devices processed using spin-coating; although this works well for testing new materials and proof-of-concept studies, it is far from industrially-relevant. One new avenue of research in the group looks at adapting existing materials and processing methods for scale-up. We are currently exploring both slot-die coating and blade coating as processing methods for organic electronic devices.
Selected Publications on Organic Electronics
- N. M. Randell, K. M. Fransishyn, and T. L. Kelly* Lewis Acid-Base Chemistry of 7-Azaisoindigo-Based Organic Semiconductors. ACS Applied Materials & Interfaces. 2017, 9, 24788-24796. [DOI: 10.1021/acsami.7b06335]
- A. Ganguly, J. Zhu, and T. L. Kelly* Effect of Cross-Conjugation on Derivatives of Benzoisoindigo, an Isoindigo Analogue with an Extended Pi-System. The Journal of Physical Chemistry C. 2017, 121, 9110-9119. [DOI: 10.1021/acs.jpcc.7b00742]
- N. M. Randell, P. C. Boutin, and T. L. Kelly* Bisisoindigo: Using a Ring Fusion Approach to Extend the Conjugation Length of Isoindigo. Journal of Materials Chemistry A. 2016, 4, 6940-6945. [DOI: 10.1039/C5TA07511D]
- K. E. Lee, L. Liu, and T. L. Kelly* Effect of Molybdenum Oxide Electronic Structure on Organic Photovoltaic Device Performance: An X-ray Absorption Spectroscopy Study. The Journal of Physical Chemistry C. 2014, 118, 27735-27741. [DOI: 10.1021/jp508972v]
- N. M. Randell, A. F. Douglas, and T. L. Kelly* 7-Azaisoindigo as a New Electron Deficient Component of Small Molecule Chromophores for Organic Solar Cells. Journal of Materials Chemistry A. 2014, 2, 1085-1092. [DOI: 10.1039/c3ta14263a]
X-ray Scattering
The Canadian Light Source, Canada's national synchrotron facility, is co-located on the University of Saskatchewan campus. With such a powerful tool available for the structural characterization of thin films and devices, we are making increasing use of X-ray scattering methods in our research. We primarily rely on grazing incidence wide angle X-ray scattering (GIWAXS), which provides information about the crystalline order and arrangement of thin films. It is useful for both conjugated organic systems, where it provides information about molecular orientation and crystallinity, and inorganic materials, where it can provide both phase identification and information on grain size and orientation.
One exciting avenue of research in the group is the use of in situ and in operando methods for the study of both organic and inorganic materials. We have used a custom-machined environmental test chamber to elucidate decomposition mechanisms in lead halide perovskites [e.g., ACS Nano, 2014; Chem. Mater., 2015], and are moving toward fully in operando measurements on working solar cells and devices.
Selected Publications on X-ray Scattering
- J. Yang, K. M. Fransishyn, and T. L. Kelly* Comparing the Effect of Mesoporous and Planar Metal Oxides on the Stability of Methylammonium Lead Iodide Thin Films. Chemistry of Materials. 2016, 28, 7344-7352. [DOI: 10.1021/acs.chemmater.6b02744]
- J. Yang, B. D. Siempelkamp, E. Mosconi, F. De Angelis, and T. L. Kelly* Origin of the Thermal Instability in CH3NH3PbI3 Thin Films Deposited on ZnO. Chemistry of Materials. 2015, 27, 4229-4236. [DOI: 10.1021/acs.chemmater.5b01598]
- J. Yang, B. D. Siempelkamp, D. Liu, and T. L. Kelly* Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in situ Techniques. ACS Nano. 2015, 9, 1955-1963. [DOI: 10.1021/nn506864k]
Plasmonics
Plasmon-Enhanced Devices
Localized surface plasmons are the collective oscillation of conduction band electrons in noble metal nanoparticles that occur in response to electromagnetic radiation. They give rise to dramatic enhancements of the electric field at the particle surface, and have been used to increase the strength of many optical phenomena (e.g., surface enhanced Raman scattering). We have taken advantage of this unique behavior to enhance the light harvesting efficiency of thin films and optoelectronic devices - effectively turning the nanoparticles into nanoscale antennas for visible light!
Applications of this approach have ranged from increasing the light harvesting efficiency of dye-sensitized solar cells [ACS Appl. Mater. Interfaces, 2013; Langmuir, 2014], to improving the triplet-yield of photon upconversion thin films [J. Phys. Chem. C, 2014]. More recent work has even used the high temperatures induced by non-radiative plasmon relaxation to drive chemistry at the nanoparticle surface [RSC Adv., 2017].
Stability and Surface Chemistry of Anisotropic Metal Nanoparticles
As part of our efforts to incorporate these nanomaterials into devices, we have had to answer fundamental questions about the stability of anisotropic metal nanoparticles and to introduce novel surface chemistries to better solubilize and/or protect the nanoparticle. Much of this work has been done with triangular silver nanoprisms, and has ranged from in situ X-ray absorption near-edge spectroscopy (XANES) and UV/vis spectroscopy studies on nanoparticle/thiol mixtures, to the step-wise functionalization of nanoparticle surfaces.
Selected Publications on Plasmonics
- M. K. Gangishetty, R. W. J. Scott, and T. L. Kelly* Thermal Degradation Mechanism of Triangular Ag@SiO2 Nanoparticles. Dalton Transactions. 2016, 45, 9827-9834. [DOI: 10.1039/C6DT00169F]
- K. E. Lee, A. V. Hesketh, and T. L. Kelly* Chemical Stability and Degradation Mechanisms of Triangular Ag, Ag@Au, and Au Nanoprisms. Physical Chemistry Chemical Physics. 2014, 16, 12407-12414. [DOI: 10.1039/C4CP00954A]
- K. Poorkazem, A. V. Hesketh, and T. L. Kelly* Plasmon-enhanced Triplet-Triplet Annihilation using Silver Nanoplates. The Journal of Physical Chemistry C. 2014, 118, 6398-6404. [DOI: 10.1021/jp412223m]
- M. K. Gangishetty, K. E. Lee, R. W. J. Scott, and T. L. Kelly* Plasmonic Enhancement of Dye Sensitized Solar Cells in the Red-to-NIR Region using Triangular Core-Shell Ag@SiO2 Nanoparticles. ACS Applied Materials & Interfaces. 2013, 5, 11044-11051. [DOI: 10.1021/am403280r]
- L. Liu, C. A. Burnyeat, R. S. Lepsenyi, I. O. Nwabuko, and T. L. Kelly* The Mechanism of Shape Evolution in Ag Nanoprisms Stabilized by Thiol-terminated Poly(ethylene glycol): an in situ Kinetic Study. Chemistry of Materials. 2013, 25, 4206-4214. [DOI: 10.1021/cm401944b]
- L. Liu, and T. L. Kelly* Phase Transfer of Triangular Silver Nanoprisms from Aqueous to Organic Solution by an Amide Coupling Reaction. Langmuir. 2013, 29, 7052-7060. [DOI: 10.1021/la4005856]
