Recent Publications
Click on the graphical abstract to view the article on the publisher's website.
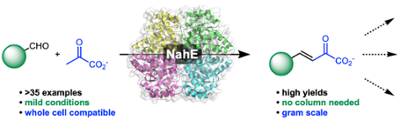
Repurposing an aldolase for the chemoenzymatic synthesis of substituted quinolines. Fansher, et al. ACS Catalysis, 2021, 11, 6939–6943.
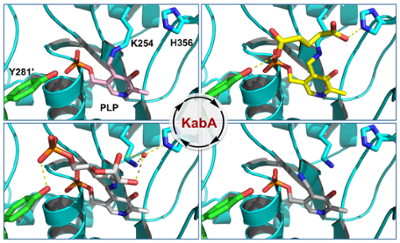
Snapshots along the catalytic path of KabA, a PLP-dependent aminotransferase required for kanosamine biosynthesis in Bacillus cereus UW85. Prasertanan et al., Journal of Structural Biology, 2021, 213, 107744.
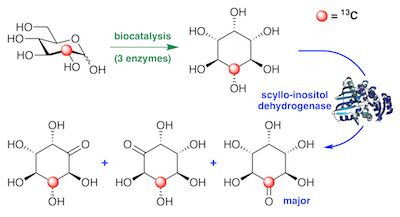
Preparation and application of 13C-labeled myo-inositol to identify new catabolic products in inositol metabolism in Lactobacillus casei. Ramos-Figueroa, et al. Biochemistry, 2020, 59, 2974–2985.
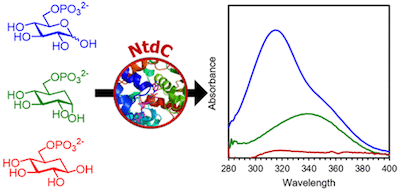
Carbocyclic substrate analogs reveal kanosamine biosynthesis begins with the alpha-anomer of glucose 6-phosphate. Vetter et al., ACS Chemical Biology, 2020, 15, 2205–2211.
Complete list of publications for David R. J. Palmer
University of Saskatchewan
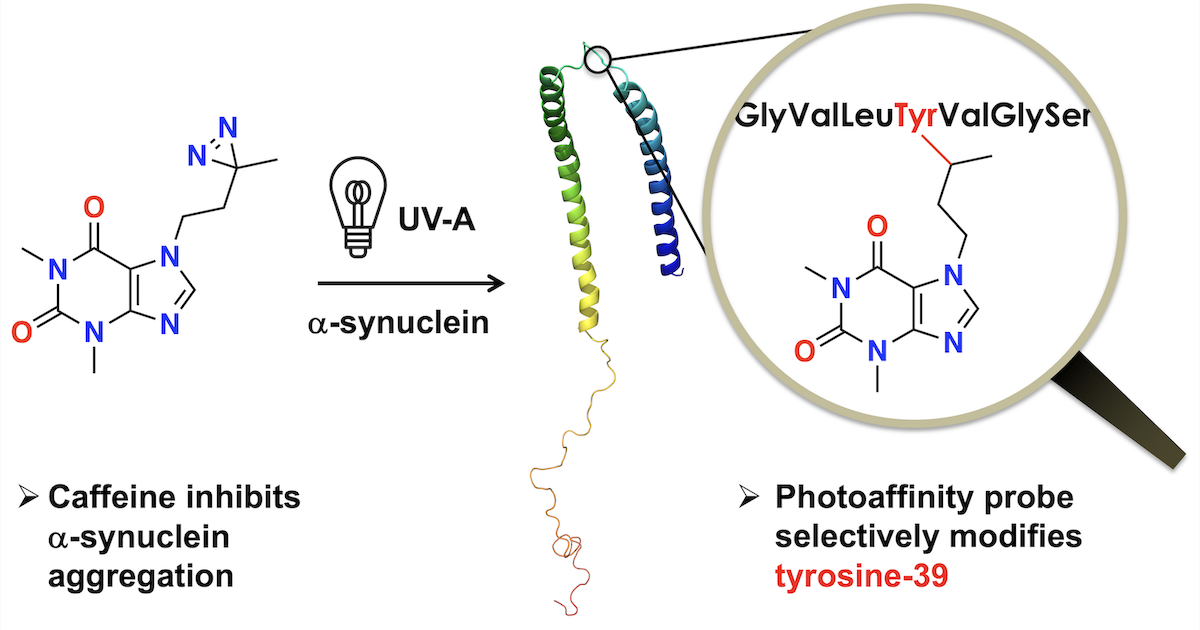
61. M. Mejia-Gutierrez, B. Moser, M. Pirlot, H. Zhang, P. Chumala, G. S. Katselis, D. R. J. Palmer, E. S. Krol* (2025) Caffeine and nicotine with N-substituted diazirine photoaffinity labels form adducts at Tyrosine-39 of α-synuclein. ACS Chemical Neuroscience, 16, 1539–1549. https://pubs.acs.org/doi/10.1021/acschemneuro.5c00074.
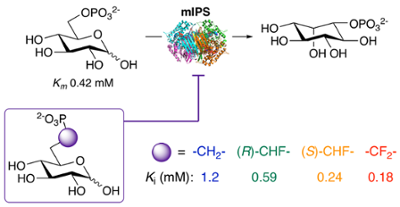
60. J. S. Ramos-Figueroa, N. D. Vetter, D. R. J. Palmer* (2023) Phosphonate and α-fluorophosphonate analogs of D-glucose 6-phosphate as active-site probes of 1L-myo-inositol 1-phosphate synthase. Methods in Enzymology, volume 685, New Experimental Probes for Enzyme Specificity and Mechanism. pp. 57–93. https://doi.org/10.1016/bs.mie.2023.03.016
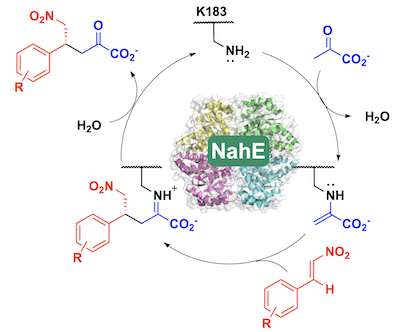
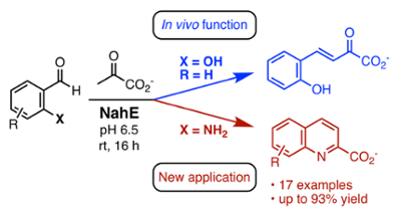
59. D. J. Fansher and D. R. J. Palmer* (2023) A Type 1 Aldolase, NahE, Catalyzes a Stereoselective Nitro-Michael Reaction: Synthesis of β-Aryl-γ-nitrobutyric Acids. Angewandte Chemie - International Edition, 62, e202214539. *Chosen by the Editors as a Hot Paper https://doi.org/10.1002/anie.202214539
58. D. J. Fansher, N. Ngwira, A. R. Salehi, J. Woods, A. Cascao, D. R. J. Palmer* (2023) Biocatalytic Synthesis of α,β-Unsaturated 2-Keto Acids and Derivatives Using the Promiscuous Aldolase NahE. Synthesis, 55, 75-89. (Feature Article) https://doi.org/10.1055/a-1953-1509
57. D. R. J. Palmer (2022) Biocatalysis tips another domino: a single-enzyme Enders triple cascade reaction. Chem Catalysis, 2, 2452-2454. (Invited Preview) https://www.cell.com/chem-catalysis/fulltext/S2667-1093(22)00527-9
56. J. S. Ramos-Figueroa*, D. R. J. Palmer, and G. P. Horsman* (2022) Phosphoenolpyruvate mutase-catalyzed C-P bond formation: mechanistic ambiguities and opportunities. ChemBioChem, 23, e202200285. https://doi.org/10.1002/cbic.202200285
55. J. S. Ramos-Figueroa and D. R. J. Palmer* (2022) Phosphonate and α-fluorophosphonate analogs of D-glucose 6-phosphate as active-site probes of 1L-myo-inositol 1-phosphate synthase. Biochemistry, 61, 868-878. link
54. P. Bhandari, J. P. Tingley, D. R. J. Palmer, D. W. Abbott and J. E. Hill* (2021) Characterization of an α-glucosidase enzyme conserved in Gardnerella spp. isolated from the human vaginal microbiome. Journal of Bacteriology, 203, e00213-21. link *Highlighted by the Editors as an Article of Significant Interest link
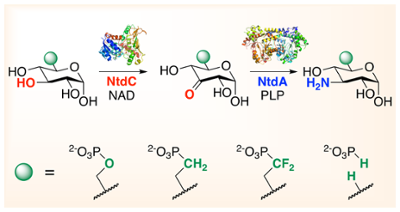
53. N. D. Vetter and D. R. J. Palmer* (2021) Substrate substitution in kanosamine biosynthesis using phosphonates and phosphite rescue. Biochemistry, 60, 1926–1932. link
52. D. J. Fansher, R. Granger, S. Kaur, and D. R. J. Palmer* (2021) Repurposing an aldolase for the chemoenzymatic synthesis of substituted quinolines. ACS Catalysis, 11, 6939–6943. link
51. T. Prasertanan, D. R. J. Palmer*, and D. A. R. Sanders* (2021) Snapshots along the catalytic path of KabA, a PLP-dependent aminotransferase required for kanosamine biosynthesis in Bacillus cereus UW85. Journal of Structural Biology, 213, 107744. link
50. J. S. Ramos-Figueroa, H. B. Aamudalapalli, R. C. Jagdhane, J. Smith, and D. R. J. Palmer* (2020) Preparation and application of 13C-labeled myo-inositol to identify new catabolic products in inositol metabolism in Lactobacillus casei. Biochemistry, 59, 2974-2985. link
49. N. D. Vetter, R. C. Jagdhane, B. J. Richter, and D. R. J. Palmer* (2020) Carbocyclic substrate analogs reveal kanosamine biosynthesis begins with the alpha-anomer of glucose 6-phosphate. ACS Chemical Biology, 15, 2205-2211. link
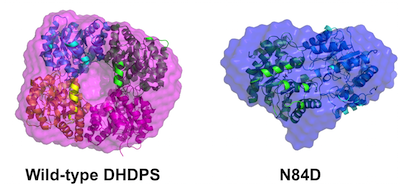
48. M. Majdi Yazdi, S. Saran, T. Mrozowich, C. Lehnert, T. R. Patel*, D. A. R. Sanders*, and D. R. J. Palmer* (2020) Asparagine-84, a regulatory allosteric site residue, helps maintain the quaternary structure of Campylobacter jejuni dihydrodipicolinate synthase. Journal of Structural Biology, 209, 107409. link
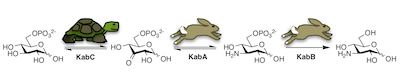
47. T. Prasertanan and D. R. J. Palmer* (2019) The kanosamine biosynthetic pathway in Bacillus cereus UW85: functional and kinetic characterization of KabA, KabB, and KabC. Archives of Biochemistry and Biophysics, 676, 108139. link
46. H. B. Aamudalapalli, D. Bertwistle, D. R. J. Palmer*, and D. A. R. Sanders* (2018) myo-Inositol dehydrogenase and scyllo-inositol dehydrogenase from Lactobacillus casei BL23 bind their substrates in very different orientations. BBA - Proteins and Proteomics, 1866, 1115-1124. link
45. N. D. Vetter and D. R. J. Palmer* (2017) Simultaneous measurement of glucose-6-phosphate 3-dehydrogenase (NtdC) catalysis and the nonenzymatic reaction of its product: kinetics and isotope effects on the first step in kanosamine biosynthesis. Biochemistry, 56, 2001-2009. link
44. M. A. Sowole, S. Simpson, Y. V. Skovpen, D. R. J. Palmer, and L. Konermann* (2016) Evidence for Allosteric Enzyme Regulation via Changes in Conformational Dynamics: An H/D Exchange Investigation of Dihydrodipicolinate Synthase. Biochemistry, 55, 5413-5422. link
43. Y. V. Skovpen, C. J. T. Conly, D. A. R. Sanders* and D. R. J. Palmer* (2016) Biomimetic design results in a potent inhibitor of dihydrodipicolinate synthase from Campylobacter jejuni. Journal of the American Chemical Society, 138, 2014-2020. link
* Highlighted in JACS Spotlights link
42. M. M. Galka, N. Rajagopalan, L. M. Buhrow, K. M. Nelson, J. Switala, A. J. Cutler, D. R. J. Palmer, P. C. Loewen, S. R. Abrams, and M. C. Loewen* (2015) Identification of Interactions between Abscisic Acid and Ribulose-1,5-bisphosphate Carboxylase/Oxygenase. PLOS One, 10(7): e0133033. link
41. S. M. Forget, A. Jee, D. A. Smithen, R. Jagdhane, S. Anjum, S. A. Beaton, D. R. J. Palmer, R. T. Syvitski, and D. L. Jakeman* (2015) Kinetic evaluation of glucose 1-phosphate analogues with a thymidylyltransferase using a continuous coupled enzyme assay. Organic and Biomolecular Chemistry, 13, 866-875. link
40. C. J. T. Conly, Y. V. Skovpen, S. Li, D. R. J. Palmer* and D. A. R. Sanders* (2014) Tyrosine 110 Plays a Critical Role in Regulating the Allosteric Inhibition of Campylobacter jejuni Dihydrodipicolinate Synthase by Lysine. Biochemistry, 53, 7067-7075. link
39. D. Bertwistle, L. Vogt, H. B. Aamudalapalli, D. R. J. Palmer and D. A. R. Sanders* (2014) Purification, crystallization and room temperature x-ray diffraction of inositol dehydrogenase Iolg2 from Lactobacillus casei BL23. Acta Crystallographica F, 70, 979-983. link
38. K. E. van Straaten, J. B. Ko, R. Jagdhane, S. Anjum, D. R. J. Palmer, and D. A. R. Sanders* (2013) The structure of NtdA, a sugar aminotransferase involved in the kanosamine biosynthetic pathway in Bacillus subtilis, reveals a new sub-class of aminotransferases. Journal of Biological Chemistry, 288, 34121-34130. link
36. Y. V. Skovpen and D. R. J. Palmer* (2013) Dihydrodipicolinate synthase from Campylobacter jejuni: kinetic mechanism of cooperative allosteric inhibition and inhibitor-induced substrate cooperativity. Biochemistry, 52, 5454-5462. link
35. N. D. Vetter, D. M. Langill, S. Anjum, J. Boisvert-Martel, R. C. Jagdhane, E. Omene, H. Zheng, K. E. van Straaten, I. Asiamah, E. S. Krol, D. A. R. Sanders, and D. R. J. Palmer* (2013) A Previously-Unrecognized Kanosamine Biosynthesis Pathway in Bacillus subtilis. Journal of the American Chemical Society, 135, 5970-5973. link
* Highlighted in JACS Spotlights link
34. I. Gabriel*, N. D. Vetter, D. R. J. Palmer, M. Milewska, M. Wojciechowski, S. Milewski (2013) Homoisocitrate dehydrogenase from Candida albicans: properties, inhibition and targeting by an antifungal pro-drug. FEMS Yeast Research, 13, 143-155. link
33. S. Anjum, N. D. Vetter, J. E. Rubin, and D. R. J. Palmer* (2013) Synthesis of 3,3'-Neotrehalosadiamine and related 1,1'-Aminodisaccharides Using Disarmed, Armed, and Super Armed Building Blocks. Tetrahedron, 69, 816-825. link
32. M. Fang, A. Macova, K. L. Hanson, J. Kos, and D. R. J. Palmer* (2011) Using substrate analogues to probe the kinetic mechanism and active site of Escherichia coli MenD. Biochemistry, 50, 8712-8721. link
31. K. E. van Straaten, H. Zheng, D. R. J. Palmer, and D. A. R. Sanders* (2010) Structural Investigation of myo-Inositol Dehydrogenase from Bacillus subtilis: Implications for Catalytic Mechanism and Inositol Dehydrogenase Subfamily Classification. Biochemical Journal, 432, 237-247. link
30. M. Fang, B. M. Langman, and D. R. J. Palmer* (2010) A stable analogue of isochorismate for the study of MenD and other isochorismate-utilizing enzymes. Bioorganic and Medicinal Chemistry Letters, 20, 5019-5022. link
29. M. Fang, R. D. Toogood, A. Macova, K. Ho, S. G. Franzblau, M. R. McNeil, D. A. R. Sanders, and D. R. J. Palmer* (2010) Succinylphosphonate esters are competitive inhibitors of MenD that show active-site discrimination between homologous alpha-ketoglutarate-decarboxylating enzymes. Biochemistry, 49, 2672-2679. link
28. K. E. Van Straaten, D. M. Langill, D. R. J. Palmer and D. A. R. Sanders* (2009) Purification, crystallization and preliminary X-ray analysis of NtdA, a pyridoxal phosphate-dependent aminotransferase from Bacillus subtilis. Acta Crystallographica F, 65, 426-429. link
27. R. M. Drevland, Y. Jia, D. R. J. Palmer, and D. E. Graham* (2008) Methanogen homoaconitase catalyzes both hydro-lyase reactions in coenzyme B biosynthesis. Journal of Biological Chemistry, 283, 28888-28896. link
26. C. P. Phenix and D. R. J. Palmer* (2008) Isothermal titration microcalorimetry reveals the cooperative and noncompetitive nature of inhibition of Sinorhizobium meliloti L5-30 dihydrodipicolinate synthase by (S)-lysine. Biochemistry, 47, 7779-7781. link
25. C. P. Phenix, K. Nienaber, P. H. Tam, L. T. J. Delbaere, and D. R. J. Palmer* (2008) Structural, functional and calorimetric investigation of MosA, a dihydrodipicolinate synthase from Sinorhizobium meliloti L5-30, does not support involvement in rhizopine biosynthesis. ChemBioChem, 9, 1591-1602. link
24. K. E. Van Straaten, A. Hoffart, D. R. J. Palmer and D. A. R. Sanders* (2008) Purification, crystallization and preliminary X-ray analysis of inositol dehydrogenase (IDH) from Bacillus subtilis. Acta Crystallographica F, 64, 98-101. link
23. R. Daniellou, H. Zheng, D. M. Langill, D. A. R. Sanders and D.R.J. Palmer* (2007) Probing the Promiscuous Active Site of myo-Inositol Dehydrogenase using Synthetic Substrates, Homology Modelling, and Active Site Modification. Biochemistry, 46, 7469-7477. link
22. R. Daniellou, J. W. Quail and D. R. J. Palmer* (2006) 4/6-O-(p-Tolylsulfonyl)-myo-inositol. Acta Crystallographica E, 62, o4880-o4881. link
21. R. Daniellou and D. R. J. Palmer* (2006) Appel-Lee synthesis of glycosyl inositols, substrates for inositol dehydrogenase from Bacillus subtilis. Carbohydrate Research, 341, 2145-2150. link
20. Y. Jia, T. Tomeo, K. Yamauchi, M. Nishiyama, and D. R. J. Palmer* (2006) Kinetics and product analysis of the reaction catalyzed by recombinant homoaconitase from Thermus thermophilus. Biochemical Journal, 396, 479-485. link
19. R. Daniellou, H. Zheng, and D. R. J. Palmer* (2006) Kinetics of the reaction catalyzed by inositol dehydrogenase from Bacillus subtilis and inhibition by fluorinated substrate analogs. Canadian Journal of Chemistry, 84, 522-527. link
18. Y. A. Leduc, C. P. Phenix, J. Puttick, K. Nienaber, D. R. J. Palmer, and L. T. J. Delbaere* (2006) Crystallization, X-ray diffraction, and sturcture solution of MosA, a dihydrodipicolinate synthase from Sinorhizobium meliloti L5-30. Acta Crystallographica F, 62, 49-51. link
17. Y. Jia, D. R. J. Palmer, and J. W. Quail* (2005) The mono-(S)-alpha-methylbenzylammonium salt of (R)-homocitric lactone. Acta Crystallographica E, 61, o4034-o4036. link
16. E. A. L. Sieminska, A. Macova, D. R. J. Palmer and D. A. R. Sanders* (2005). Crystallization and preliminary X-ray analysis of (1R,6R)-2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate (SHCHC) synthase (MenD) from Escherichia coli. Acta Crystallographica F, 61, 489-492. link
15. R. Daniellou, C. P. Phenix, P. H. Tam, M. C. Laliberte, D. R. J. Palmer* (2005) Stereoselective oxidation of protected inositol derivatives catalyzed by inositol dehydrogenase from Bacillus subtilis. Organic and Biomolecular Chemistry, 3, 401-403. link
14. D. R. J. Palmer* (2004). Integration of computational and preparative techniques to demonstrate physical organic concepts in synthetic organic chemistry: an example using Diels-Alder reactions. Journal of Chemical Education, 81, 1633-1635. link
13. P. H. Tam, C. P. Phenix, D. R. J. Palmer* (2004). MosA, a protein implicated in rhizopine biosynthesis in Sinorhizobium meliloti L5-30, is a dihydrodipicolinate synthase. Journal of Molecular Biology, 335, 393-397. link
12. D. R. J. Palmer,* H.N. Balogh, G. Ma, X. Zhou, M. Marko, S.G.W. Kaminskyj* (2004). Synthesis and anti-fungal properties of novel compounds which target the alpha-aminoadipate pathway. Die Pharmazie 59, 93-98. link
11. M. Bhasin, J. L. Billinsky, D. R. J. Palmer* (2003). Steady-state kinetics and molecular evolution of Escherichia coli MenD, or (1R,6R)-2-succinyl-6-hydroxy-2,4-cyclohexadiene synthase, and anomalous thiamin diphosphate-dependent decarboxylase - carboligase. Biochemistry, 42, 13496 - 13504. link
10. G. Ma and D. R. J. Palmer* (2000). Improved asymmetric syntheses of (R)-(-)-homocitrate and (2R,3S)-(-)-homoisocitrate, intermediates in the alpha-aminoadipate pathway of fungi. Tetrahedron Letters 41, 9209-9212. link
Prior to the University of Saskatchewan
9. E. A. Taylor, D. R. J. Palmer, J. A. Gerlt* (2001). The lesser ‘burden borne’ by o-succinylbenzoate synthase: an ‘easy’ reaction involving a carboxylate carbon acid. Journal of the American Chemical Society 123, 5824-5825.
8. D. R. J. Palmer, J. B. Garrett, V. Sharma, R. Meganathan, P. C. Babbitt, J. A. Gerlt* (1999). Unexpected divergence of enzyme function and sequence: “N-acylamino acid racemase” is ortho-succinylbenzoate synthase. Biochemistry 38, 4252-4258.
7. D. R. J. Palmer, B. K. Hubbard, and J .A. Gerlt* (1998). Evolution of enzymatic activities in the enolase superfamily: partitioning of reactive intermediates by glucarate dehydratase from Pseudomonas putida. Biochemistry 37, 14350-14357.
6. A. Gulick, D. R. J. Palmer, P. C. Babbitt, J. A. Gerlt and I. Rayment* (1998). Evolution of enzymatic activities in the enolase superfamily: crystal structure of D-glucarate dehydratase from Pseudomonas putida. Biochemistry 37, 14358-14368.
5. B. K. Hubbard, M. Koch, D. R. J. Palmer, P. C. Babbitt, and J. A. Gerlt* (1998). Evolution of enzymatic activities in the enolase superfamily: characterization of the D-glucarate/galactarate catabolic pathway in Escherichia coli. Biochemistry 37, 14369-75.
4. D. R. J. Palmer, S. J. Wieczorek, B. K. Hubbard, G. T. Mrachko and J. A. Gerlt* (1997). Importance of mechanistic imperatives in enzyme-catalyzed beta-elimination reactions: Stereochemical consequences of dehydration reactions catalyzed by galactonate dehydratase from Escherichia coli and glucarate dehydratase from Pseudomonas putida. Journal of the American Chemical Society 119, 9580-9581.
3. P. C. Babbitt,* M. S. Hasson, J. E. Wedekind, D. R. J. Palmer, W. C. Barrett, G. H. Reed, I. Rayment, D. Ringe, G. L. Kenyon, J. A. Gerlt* (1996), The enolase superfamily: a general strategy for enzyme-catalyzed abstraction of the alpha-protons of carboxylic acids. Biochemistry 35, 16489-16501.
2. D. R. J. Palmer and J. A. Gerlt* (1996), Evolution of enzymatic activities: multiple pathways for generating and partitioning of a common enolic intermediate by glucarate dehydratase from Pseudomonas putida. Journal of the American Chemical Society 118, 10323-10324.
1. D. R. J. Palmer, E. Buncel, G. R. J. Thatcher* (1994) Re-evaluation of cyclodextrin as a model of chymotrypsin: acceleration and inhibition of tertiary anilide hydrolysis. Journal of Organic Chemistry 59, 5286-5291.