What we offer
Pre-clinical to Clinical trials
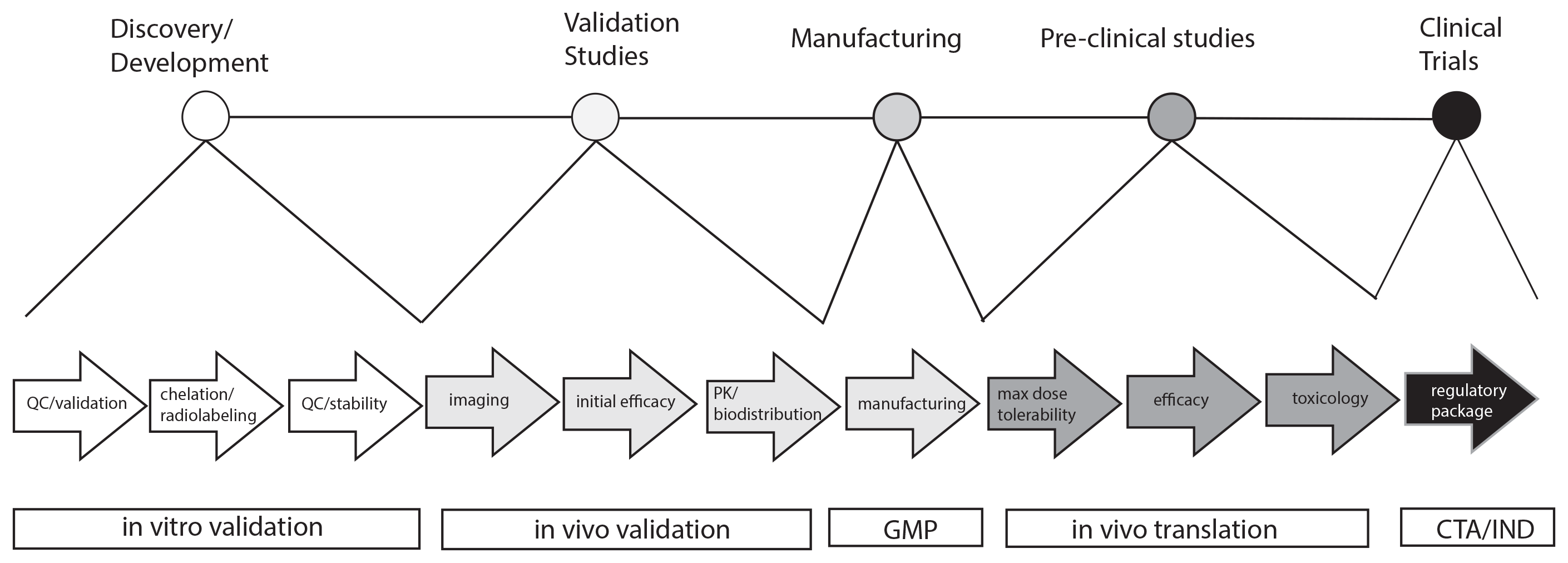
We have access to radiochemistry and animal facilities for nuclear probe development including several locally produced radioisotopes. In addition to nuclear imaging, we also develop optical probes and can provide fluorescence and luminescence imaging services. The services are available to researchers at U of S and the greater research community including other academic institutes and industry. In addition to these services we offer supporting translational activities including dosimetry, toxicity (CBC and Clnical Chemistry, histopathology), biodistribution, and efficacy studies. We also offer support in GMP formulaton, regulatory filings and intellecual property filing through the University of Saskatchewan. Finally we can support early phase clinical trial execution.
Resources and Services
Consulting
- Experience in regulatory and preclinical development.
Reporting
- Reproducible workflows: All reports are generated from source data using scripted R pipelines, ensuring transparency, consistency, and traceability. Robust error detection and data validation are used to identify potential human data entry errors.
- Immutable source data: R scripts are used for data cleaning, transformation, and validation to minimize human error and maintain data integrity. Source datasets remain unaltered.
- Standardized, automated reporting: Reports are dynamically generated using R Markdown or Bookdown templates for consistency and flexability to suit a clients needs: reporting is available as docx, pdf, or interactive html outputs. Client color profiles and preffered graphing themes can be easily implemented to generate graphs consistant with thier branding. Raw datasets for custom plotting are available as csv or xlsx.
Probe Development
- Conjugation of fluorescent moeity (GLP or GMP)
- Conjugation and Radiolabeling (GLP or GMP)
- Stability and Quality Control Assays (GLP or GMP)
- Scale-up and production in GMP space and preparation of supporting regulatory documents (QiS/QoS)
Probe Assays
- In Vitro Assays
- Cell-binding - Flow cytometry
- Purity and Identity - iTLC
- Purity and Identity - HPLC
- Cell-killing - Incucyte
- Imaging (see Imaging Modalities)
- Efficacy studies (see Animal Studies)
- Safety studies (see Animal Studies)
Imaging modalities:
- Combination micro PET (Positron Emission Tomography) / SPECT (Single Photon Emission computed Tomography)
- Large Animal PET/SPECT
- Clinical PET/SPECT
- Fluorescence Imaging
- Bioluminescence imaging (BLI)
Animal Studies
- Available Species
- Mouse/Rat through JAX/Charles River
- Pigs through the Prarie Swine Centre
- Other
- In-vivo
- Near infrared optical imaging (small animal)
- microPET/SPECT/CT imaging (large or small animal)
- Optical/CT imaging (large or small animal)
- Dosimetry (large or small animal)
- Efficacy/Pharmacodynamics
- Pharmacokinetics
- Ex vivo
- Histopathology and toxicity
- Subcellular/cellular localization (confocal microscopy)
- Biodistribution/Dosimetry
Clinical trials
- Preparation of regulatory filings
- Submission of regulatory filings
- Execution of Clinical Trials
Radionuclides
- Therapeutic radioisotopes (e.g. 161Tb, 225Ac, 177Lu, 67Cu; 90Y)
- SPECT radioisotope (e.g. 99mTc; 99Mo/99mTc; 111In)
- PET radioisotopes (e.g. 11C; 18F; 89Zr; 68Ga)
Radiochemistry
- PET and SPECT – GMP and non-GMP radiochemistry R&D of biologics (peptides, antibodies, proteins)
- Automated synthesis
Technology
The key elements to molecular probe development requires multidisciplinary efforts including molecular and cell biology, chemistry, nuclear, optical and acoustic reporter systems, modeling of disease, imaging and translation.
Technology Platform Core: C-BIRD has established a core to advance these technologies from preclinical development to human application through the following core competencies at the University of Saskatchewan:
- Radionuclide Production and Radiopharmaceutical Development: The Fedoruk Centre has a 24MeV cyclotron and GMP radiochemical facilities for production of medical radionuclides and radiopharmaceuticals. We have worked closely with them to manufacture our clinical trial doses and allow them to offer GMP grade Zr-89 registed with Health Canada.
- Animal Imaging: The College of Medicine houses and operates facilities with preclinical and clinical PET, SPECT, MRI, and optical, and acoustic non-invasive scanners for anatomical and functional imaging of living organisms.
- Large Animal Imaging: Western College of Veterinary Medicine (WCVM) operates a large animal vivarium and provides non-rodent animal models of diseases, which enable advances in diagnostics and drug therapies for animal and human health.
- Human Studies: Working closely with clinicians at the local hospitals and nurses from the clinical trial support unit (CTSU) we can run Human studies. We have also been working closely with the WCVM and have used the Allard-Roozen Imaging Suite (PET-CT) for human studies.
Our Process