Ontario
Regional prevalence data for gastrointestinal parasites, heartworm, fleas, and ticks in Ontario
Region-specific parasites
- Echinococcus multilocularis
Gastrointestinal parasites
Few studies have been conducted in recent years on the prevalence of intestinal parasites in dogs and cats in Ontario. Findings from the fecal examination of 70 owned dogs in the Niagara region are summarized below (Shukla et al 2006).
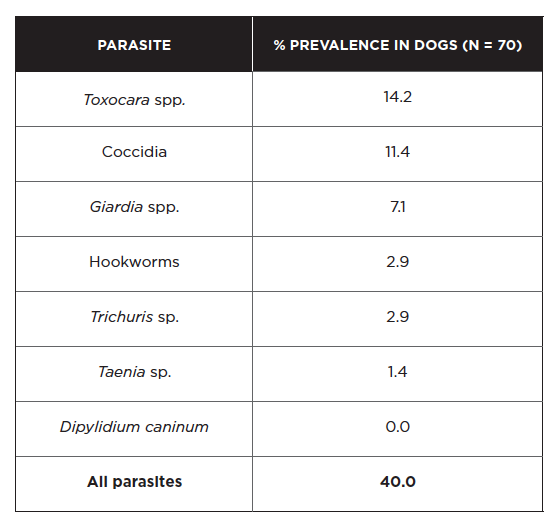
Overall, parasite prevalence for all species detected was higher in dogs 6 months of age or younger compared to dogs > 6 months of age, with the exception of Taenia and Trichuris. Cryptosporidium antigen was detected in 5/68 dogs (7.4%) based on an enzyme-linked immunosorbent assay that does not differentiate between species (Shukla et al 2006). A separate investigation identified Giardia spp. in 16/251 (6.4%) fecal samples collected from dog parks in southwestern Ontario (Procter et al 2014), consistent with the prevalence observed in the first study. Giardia cysts shed by pet dogs were determined to be mainly of the non-zoonotic C and D assemblages in one study, though 1 sample out of 75 contained a potentially zoonotic B assemblage (McDowall et al 2011).
Dogs can also be infected with Echinococcus granulosus and E. multilocularis, although rarely diagnosed in part because the eggs are indistinguishable from each other and from the eggs of Taenia species. It is important to note that both species of Echinococcus are zoonotic. E. granulosus / E. canadensis eggs were detected by PCR in the feces of a small number of dogs from Ontario shelters (Villeneuve et al 2015). Of note, canine alveolar hydatid disease due to E. multilocularis – with dogs acting as the intermediate host rather than the definitive host – has been diagnosed in Ontario’s Golden Horseshoe area in recent years (Peregrine 2015). This parasite is highly prevalent in the fox and coyote population in Ontario, and with coyotes becoming increasingly urban, the risk of exposure for dogs and people is likely to rise. In order to mitigate the danger of alveolar hydatidosis, dogs should not be allowed access to fox or coyote feces. Dogs that ingest rodents are at risk of developing intestinal infections with E. multilocularis and constitute a public health concern. High-risk dogs with rodent access should be treated monthly with praziquantel, year-round. Owners should practice good general hygiene.
Findings from the fecal examination of 41 owned cats in the Niagara region are summarized below (Shukla et al 2006).
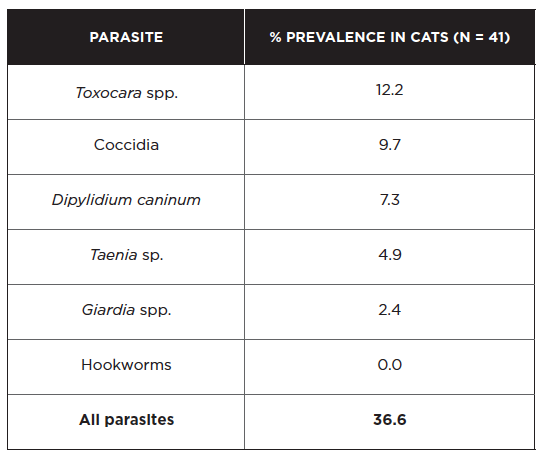
Toxocara and coccidia were much more commonly detected in cats 6 months of age or younger compared to cats > 6 months of age. Cryptosporidium antigen was detected in 3/41 cats (7.3%) based on an enzyme-linked immunosorbent assay that does not differentiate between species (Shukla et al 2006). Unlike dogs, 13/13 pet cats in a Giardia genotyping study were infected with potentially zoonotic assemblages A or B (McDowall et al 2011). Another zoonotic protozoan parasite infecting cats is Toxoplasma gondii. Its short period of oocyst shedding (just 1-2 weeks), along with limited access to prey for indoor cats, may explain why it was not detected in the pets examined above. Though uncommon, extraintestinal toxoplasmosis causing pneumonia and various other manifestations does occur in cats. From 2008-2014, nine cases of disseminated toxoplasmosis were confirmed by the Animal Health Laboratory in Guelph, most of them in young male cats (Cohen et al 2016).
Heartworm
The heartworm endemic area is in southern Ontario. However, the risk of infection is greatest south of the 403/402/401 highways that run between Sarnia and Hamilton where 80% of all the Ontario cases occur. Monthly preventives should be administered from June 1st to November 1st. The overall prevalence of heartworm in Ontario dogs appears to be approximately 0.5%. However, in a few discrete areas within southern Ontario the prevalence of infection in dogs not on a heartworm preventive can be as high as 5% to 10%.
The last systematic postal survey of heartworm in Canada was carried out in 2010. Of the 564 canine cases diagnosed, 431 (76%) were in Ontario. The survey data for Ontario are presented below alongside a similar survey from 2002 and IDEXX SNAP 4Dx data from 2013-2014 (Herrin et al 2017). Because the 2013-2014 SNAP 4Dx test results are not accompanied by information on confirmatory testing, we cannot draw firm conclusions when comparing these data to veterinarian-diagnosed cases from prior years. However, it does provide an estimate of heartworm prevalence that can help to establish a temporal trend.
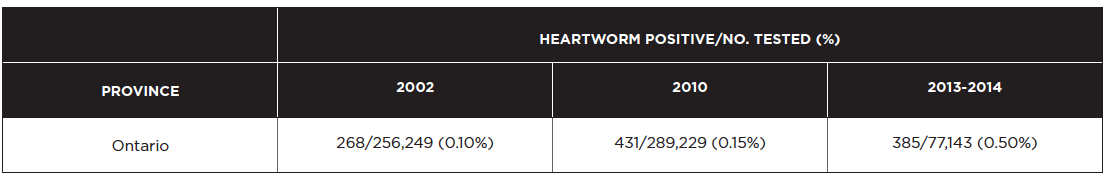
The prevalence of heartworm infection in Ontario appears to have increased since 2002. Part of this rise can be attributed to infected dogs being imported from other countries or Ontario dogs traveling abroad (approximately 25% of heartworm cases in 2010). However, half of the dogs diagnosed with heartworm infection in 2010 had never left their local area. Climate change may be allowing for the geographic expansion of mosquito vectors and thus enhancing heartworm transmission. Since no patient history is provided for the 2013-2014 SNAP 4Dx data, it is unknown if the dogs testing heartworm positive were imported from other countries or had traveled to endemic countries. While not all heartworm cases may be the result of local transmission, the fact remains that a higher prevalence of heartworm-infected dogs in a region will increase the risk of transmission to other dogs in that area.
Of note, there has been at least one report of a heartworm-infected dog in Ontario – imported as a rescue dog from New Orleans after Hurricane Katrina – whose microfilariae were highly resistant to the macrocyclic lactone class (Bourguinat et al 2011); other such resistant strains of heartworm may be out there and veterinarians should remain vigilant in monitoring their patients for heartworm infection, even those receiving monthly preventives. In particular, when treating heartworm infections, veterinarians should ensure that microfilariae are eliminated following successful adulticide treatment.
Fleas
There are no prevalence data for fleas in Ontario, though based on their experience, veterinary practitioners assess the prevalence as relatively high.
Fleas are not typically a problem year-round. The at-risk period is typically May to October, though some households in the Toronto area have experienced flea problems throughout the year.
Ticks
The most common tick seen on dogs is highly dependent on region and may be either Dermacentor variabilis (the “American dog tick”) and/or Ixodes scapularis (the “deer” or “black-legged” tick). The peak season for preventive treatment for adult ticks is typically May to July for both ticks, and also September to November for I. scapularis. Nymphs are active during the summer months and are considered an important source of B. burgdorferi infections in people. In areas endemic for I. scapularis, prevention is recommended any time the temperature is above 4°C, which may be year-round.
Annual maps of estimated Lyme disease risk areas are generated by Public Health Ontario and are available at www.publichealthontario.ca/lymedisease. The range of I. scapularis is progressively extending northward and along the coasts of Lake Ontario and Lake Erie (Nelder et al 2014). The current risk map shows areas of risk throughout eastern Ontario, in addition to the endemic foci of Point Pelee, Rondeau, Long Point, Turkey Point, Prince Edward County, and the Thousand Islands. For regions close to enzootic areas of the United States that are not yet enzootic for B. burgdorferi themselves, the main source of I. scapularis is adventitious ticks (e.g. ticks dropping from migrating birds) – mainly nymphs.
Amblyomma americanum (the “lonestar tick”) has not yet become established in Ontario, but these ticks are occasionally reported on dogs that have not traveled outside of the province (Herrin et al 2017). It is anticipated that this tick will become established in the province in the near future.
Over the years, Public Health Ontario Laboratory (PHOL) has carried out passive surveillance of Ixodes scapularis that have been submitted to them by the public. Submitted ticks are examined for the presence of both Borrelia burgdorferi and Anaplasma phagocytophilum using molecular diagnostic tests. From 2008-2012, PHOL received 5,763 I. scapularis adults. The percentage infected with B. burgdorferi increased steadily from 8.4% in 2008 to 19.1% in 2012 (overall average of 15.1%), while the percentage infected with A. phagocytophilum remained relatively unchanged with an overall average of 0.3% (Nelder et al 2014). Of the B. burgdorferi-infected ticks, 146 were fed to the point where transmission would have occurred. Therefore, only 146 (2.5%) of 5,763 tick encounters could have resulted in exposure to B. burgdorferi, suggesting the risk of dog exposure to ticks infected with B. burgdorferi is low in non-endemic areas of Ontario. The risk of exposure is higher in tick-endemic areas such as Long Point and the Thousand Islands. A recent report documented a 73% B. burgdorferi infection prevalence in I. scapularis ticks on Corkscrew Island – in northwestern Ontario’s Lake of the Woods – the northernmost location in Ontario where black-legged ticks have become established (Scott et al 2016).
The incidence of tick-borne diseases in dogs that have not traveled outside Ontario is very low. Data from a total of 115,636 Canadian dogs tested in 2013-2014 (Herrin et al 2017), compared to 94,928 samples in 2007 (from Incidence of Heartworm, Ehrlichia canis, Lyme Disease, Anaplasmosis in dogs across Canada as determined by the IDEXX SNAP® 3Dx® and 4Dx® Tests – 2007 National Incidence Study Results, IDEXX, Markham, ON) indicate that seroprevalence for tick-borne agents was as follows:
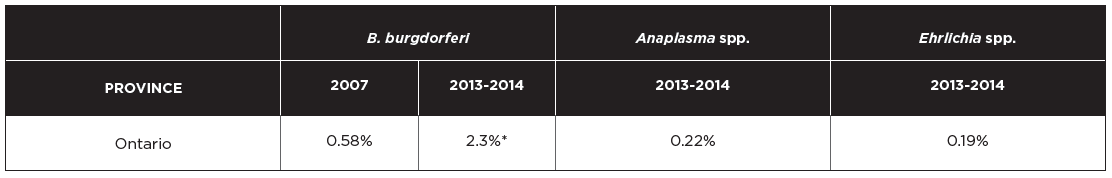
*A hyperendemic focus exists in eastern Ontario, where seroprevalence reaches 5.1%.
Because travel history and confirmatory testing were not provided for these dogs, the true prevalence of antibodies to these two pathogens in non-traveling dogs is likely lower than these figures. Furthermore, since many dogs seroconvert to tick-borne pathogens and do not develop clinical disease, the overall incidence of disease in Ontario caused by these agents is likely much lower. It is worth noting, however, that B. burgdorferi seroprevalence in dogs has increased almost four-fold in the span of 7-8 years, likely reflecting the increasing infection prevalence in I. scapularis ticks during this period. Over time, as I. scapularis populations become more established in previously non-endemic areas and more commonly infected with B. burgdorferi, the risk to dogs is expected to rise.
The Ontario Animal Health Network has generated a useful infographic to help veterinarians explain Lyme disease risk to pet owners:
For guidelines on screening dogs for Lyme disease and management of cases, see the latest ACVIM consensus statement (Littman et al 2018).
