Reactivity at Ice Surfaces
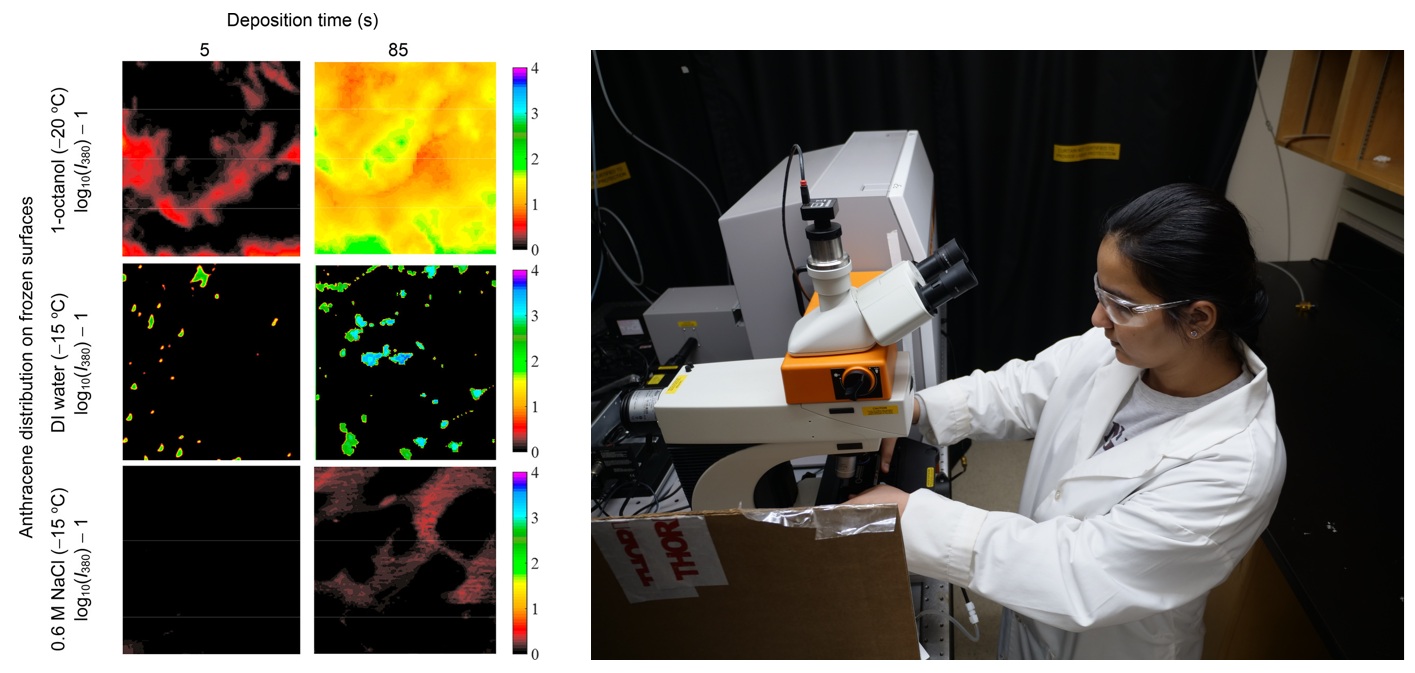
Ice covers a large part of the Earth for at least some portion of the year. Chemistry in ice can be very important to water and air quality, but little is known about reaction kinetics and mechanisms in ice. Traditional surface science techniques often can’t be used to study ice surfaces, so we develop spectro-microscopic methods to relate physical and chemical properties to reactivity.
Reactivity In Natural Waters and Aerosols
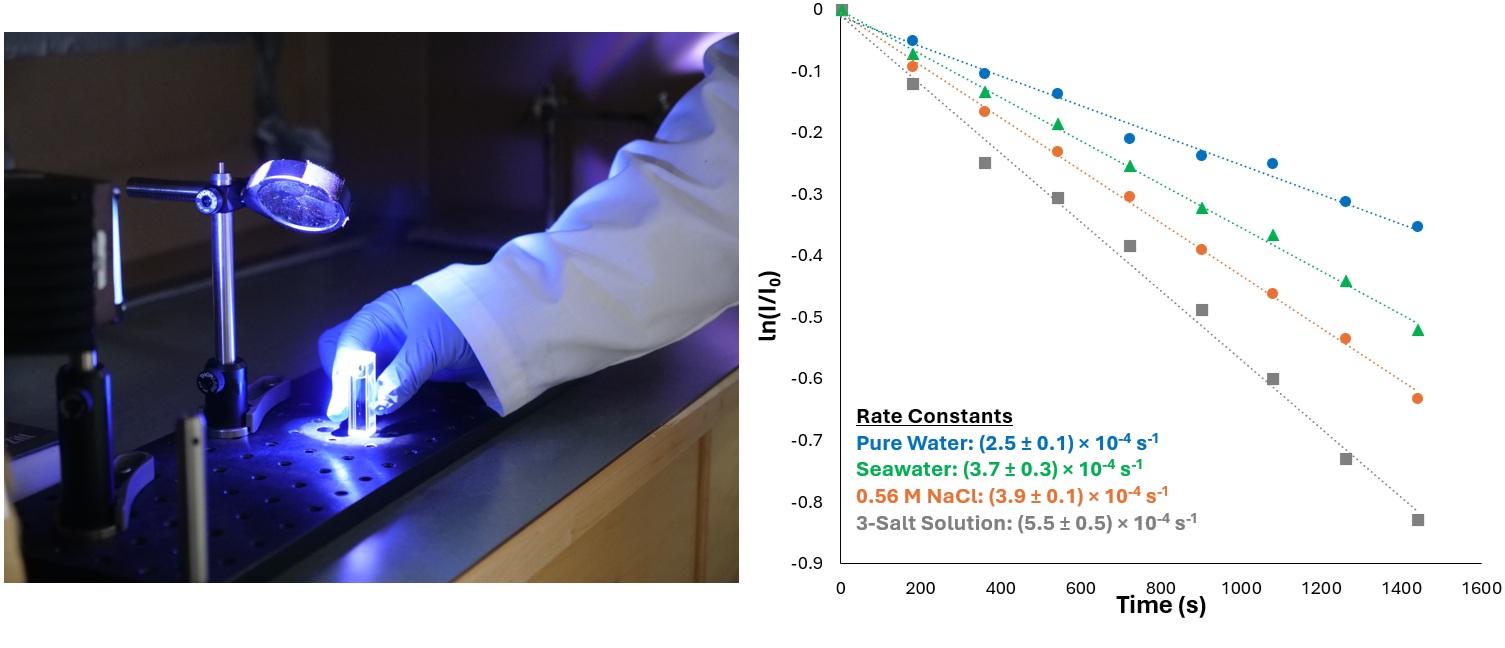
Natural waters such as oceans, lakes, and rivers are sinks for environmental pollutants and are diverse in chemical complexity and physical and chemical properties. Our work has shown that the physical and chemical properties of natural water bodies can affect the extent of chemical reactivity that occurs in them. However, their diversity makes predicting the fate of environmental pollutants difficult due to the wide range of co-solutes like salts and organic matter that can be present. This is the same for aerosols which can have very dilute or highly concentrated amounts of co-solutes. We study the effects of individual components of natural waters on the reaction kinetics of polycyclic aromatic hydrocarbons (PAHs), and compare these to reaction kinetics measured in real waters to better understand and predict the fate of organic pollutants in natural waters. We also study reaction kinetics in aerosol proxies, specifically looking at reactions in solutions with highly concentrated co-solutes (salt) to better understand reaction kinetics in aerosols and the contribution of aerosols to pollutant chemical processing.
Chemistry at Urban Surfaces (“Urban Grime”)
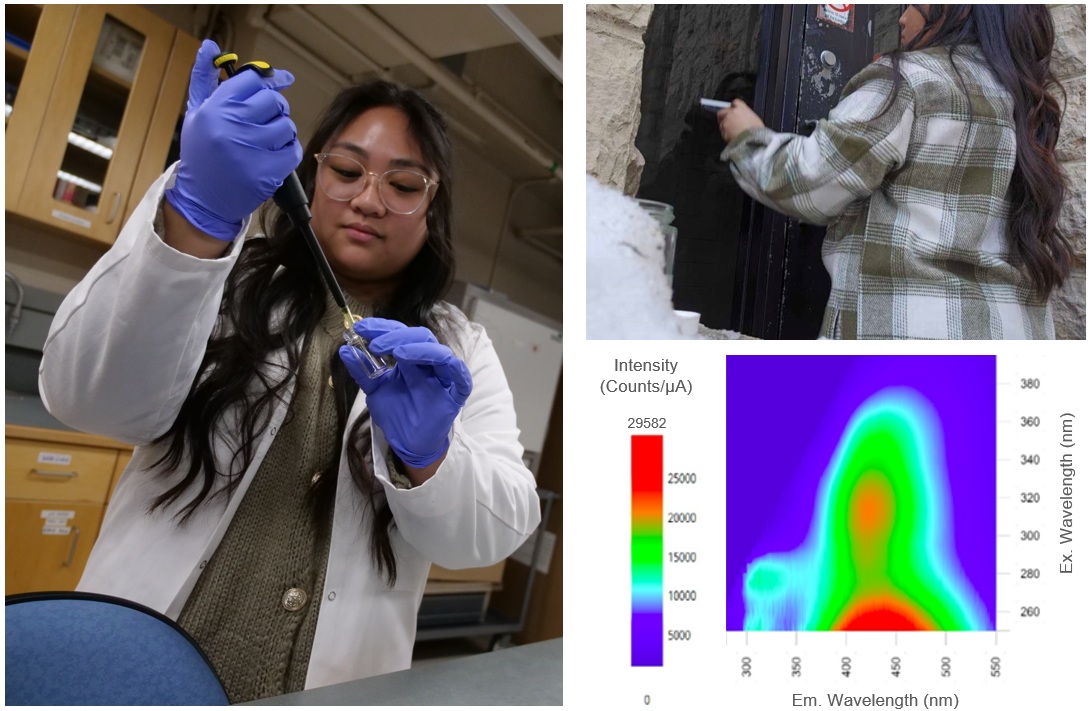
Surfaces such as roads and buildings are coated with collections of molecules and particles. These coatings may drive atmospheric processes such as partitioning and chemical processing of pollutants. However, research in this area is scarce. We use spectroscopic, microscopic, and other analytical techniques to investigate interactions between gas-phase molecules and urban grime, and to investigate the chemical transformations of anthropogenic pollutants in these coatings to determine the effects of urban grime on air and water quality in cities.
Indoor Chemistry
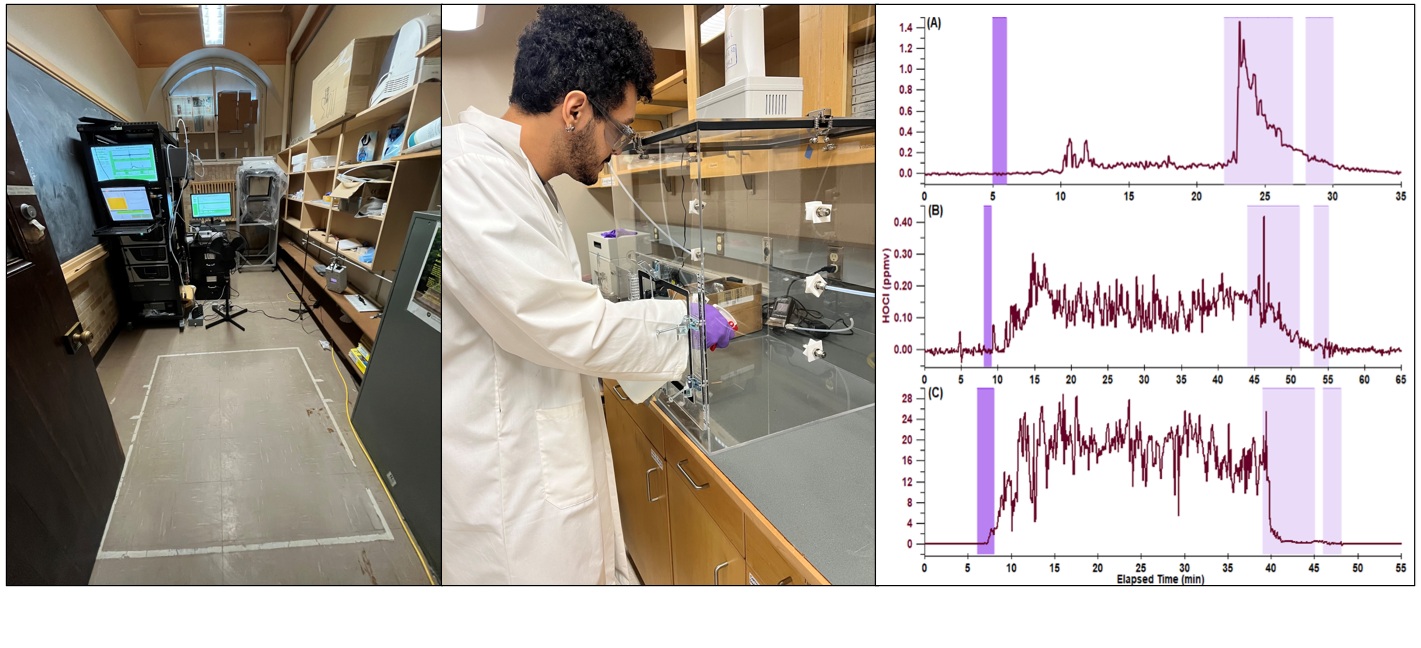
Most studies of atmospheric chemistry have focused on the outdoors, but people can spend up to 90% of their time indoors, meaning exposure to harmful compounds is likely higher indoors. A major goal in our indoor air studies is to understand how certain drivers affect indoor air quality (IAQ). We investigate the effects of human activities such as cooking and cleaning on IAQ and their potential to create reactive oxidants by measuring emissions and proposing reaction mechanisms. Key aspects of this research include studying photochemical reactions (induced by sunlight or artificial lights), making measurements in occupied spaces such as homes and offices, and developing new analytical instruments and methods.

