Missing Oxygen Atoms Are Key to Robust Spintronic Material
"the substitution of Fe atoms into In2O3 (In2O3:Fe) has recently yielded a very consistent and promising material."
Read the full article here: https://als.lbl.gov/missing-oxygen-atoms-are-key-to-robust-spintronic-material/
The endless pursuit of faster, more energy-efficient computers has led to the emergence of the field of spintronics, where the manipulation and detection of electron spins are utilized in electronic circuits. A particular class of materials called dilute magnetic oxides are promising candidates for spintronics applications because they exhibit the necessary combination of magnetic and semiconducting properties.
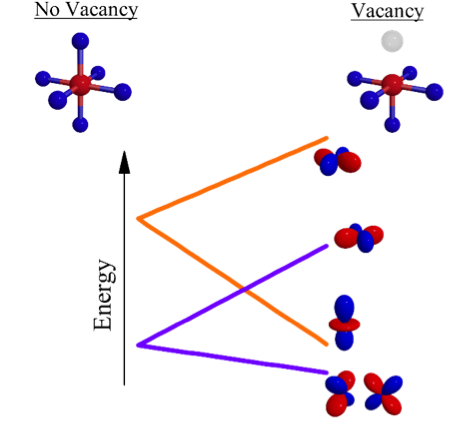
Using the soft x-ray absorption and scattering capabilities at Beamline 8.0.1, researchers studied In2O3:Fe to determine what leads to its surprisingly robust magnetic properties. They found that a subset of the impurity Fe atoms take residence in atomic sites that are directly adjacent to sites with a missing oxygen atom—the oxygen vacancies. Such positioning leads to a very strong perturbation of the Fe 3d orbital energies, which was detected in the x-ray experiments.
