Research
Price Research Group summary, and a crash-course on molecular imaging and targeted radionuclide therapy
Creating new chemical tools for the construction of next-generation molecular imaging and radionuclide therapy drugs
Eric W. Price, B.Sc., Ph.D.
Department of Chemistry, University of Saskatchewan, Saskatoon, Canada
The Price Research Group operates out of the University of Saskatchewan (USask) Chemistry Department. Group members include a diverse mixture of members including undergraduates, M.Sc. and Ph.D. students, postdoctoral fellows, and research associates. We are focused on using chemistry and radiochemistry to solve real-world problems in diagnosing and treating a variety of diseases, such as cancer. To achieve this, the Price group performs multi-/inter-disciplinary research with a primary focus in chemical synthesis and subdisciplines including organic, inorganic, bioconjugate, computational modeling, and peptide chemistry. We also actively research in the fields of radiochemistry, molecular imaging, and radionuclide therapy. We strive to find innovative solutions to real-world problems using fundamental chemical principles, including the creation of new radiopharmaceuticals and new chemical tools/reagents.

Picture Dr. Elaheh Khozeimeh Sarbisheh (left, currently at TRIUMF), Dr. Eric Price (right).
The overarching goals of the Price Research Group are to improve the chemical properties of radiopharmaceutical drugs in a modular and widely-applicable way. By creating next-generation radiopharmaceutical drugs, we are seeking to improve early detection and treatment of a variety of cancers and other diseases using molecular imaging techniques such as positron emission tomography (PET) imaging and treatment with targeted radionuclide therapy. Peptide- and antibody-based radiopharmaceuticals prepared with radioactive metal ions are the focus of the Group. These are radiopharmaceutical “smart drugs” that harness diagnostic and therapeutic radioactive metals. Once injected, these radioactive “smart drugs” can selectively seek out cancerous cells or other diseased tissue in the body by binding selectively to diseased cells that present specific receptors or biological processes. These radioactive drugs have applications in both human and animal health, and in collaboration with the Royal University Hospital and the Western College of Veterinary Medicine at the U of S, they could have a direct impact on the health of animals and the Canadian population. These radioactive “smart drugs” have the potential to become the new clinical standard for characterizing and treating diseases such as cancer, but they currently have several limitations that the Price Group aims to address (described below).
What are radiopharmaceuticals?
Radiopharmaceuticals are radioactive drugs which gain their radioactive properties by the secure chemical attachment of radioactive halogens (e.g. [131I]I-, [18F]F1-) or radioactive metal ions (radiometal, e.g. . [68Ga]Ga3+, [89Zr]Zr4+, [177Lu]Lu3+, [225Ac]Ac3+). Radiopharmaceuticals can be created from scratch as entirely novel drug products, but they are often modified from existing and previously validated drugs, peptides, proteins, antibodies, nanoparticles, or many other exotic molecular scaffolds.1 The goal for most radiopharmaceuticals is to achieve selective uptake and accumulation of radioactive elements in certain tissues (e.g., tumours, brain tissues, severe treatment resistant infections), which then enables minimally-invasive nuclear imaging or radionuclide therapy of the biological target.2 Thus, the drug portion acts as the delivery agent to provide site-specific delivery of the radionuclide, which is typically attached through chelation by a chelator (Figure 1). As drugs are often targeted very precisely to specific biological components such as cell-bound receptors, enzymes, ion-channels, transporter proteins, or certain physiochemical conditions (e.g., pH, oxygen levels), the attached radionuclide is simply a passenger that is secured to the drug and follows it to its biological target.
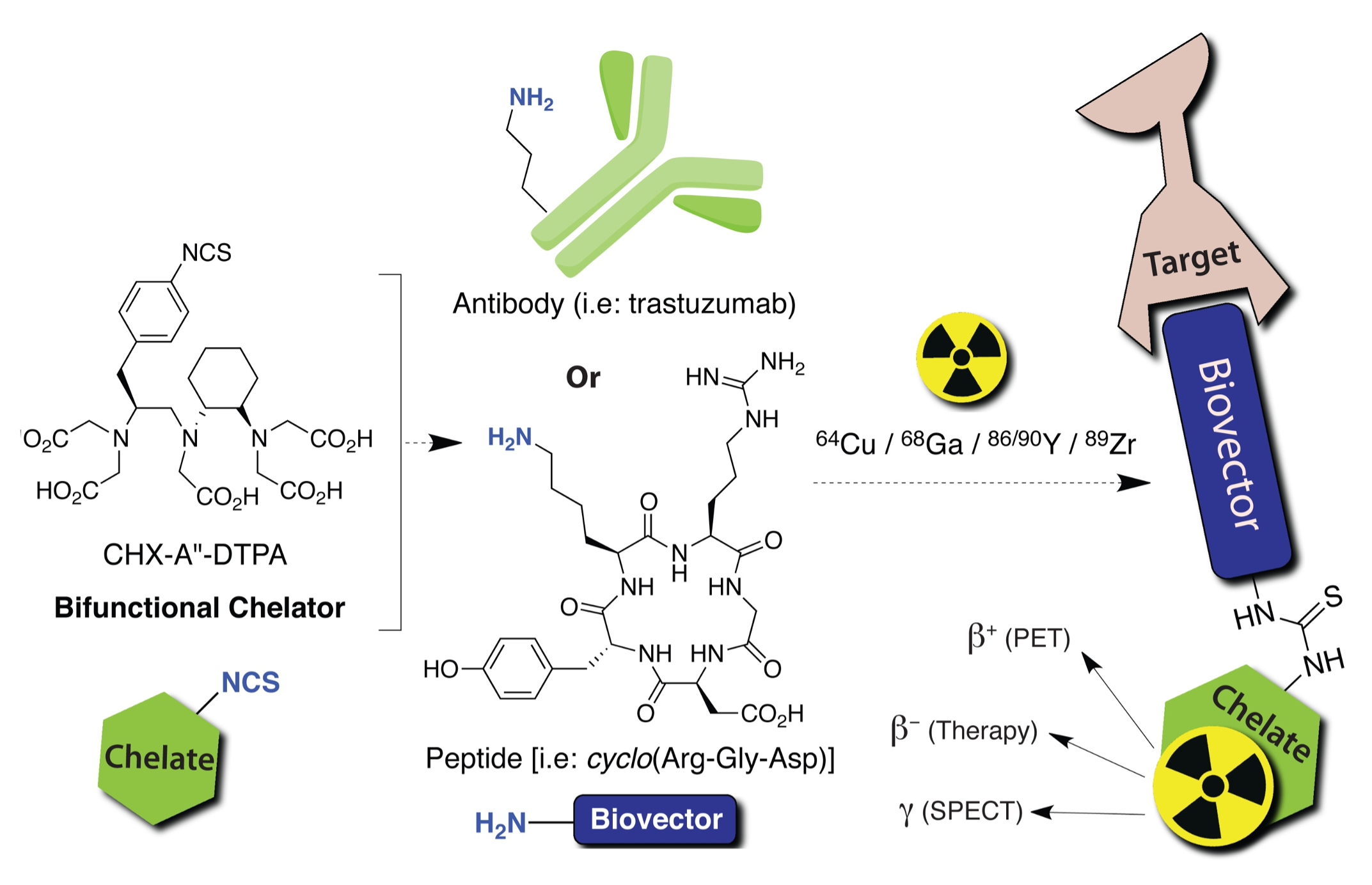
Figure 1. Cartoon illustration of a bifunctional chelate (BFC) based radiopharmaceutical agent, showing the BFC reacting with a biological targeting group such as a peptide or antibody. Figure from textbook article.3
The radiopharmaceutical is usually prepared fresh (radioactive half-life prevents long-term storage), and the drug molecule is administered to the patient in a miniscule dose that is often referred to as “sub-pharmacological”, meaning the quantity of injected chemical is too low to elicit either positive or negative biological effects (Figure 2). There might be a small risk of peptides or antibodies causing people to have harsh immune system reactions (immunogenic), particularly after multiple injections, but generally the doses are extremely small and illicit minimal reactions. The potency of radiopharmaceuticals and also the risk from them comes from the attached radionuclide and the radiation they produce. Careful dose planning and monitoring (dosimetry) has resulted in minimal cases of serious radiation damage to healthy tissues.
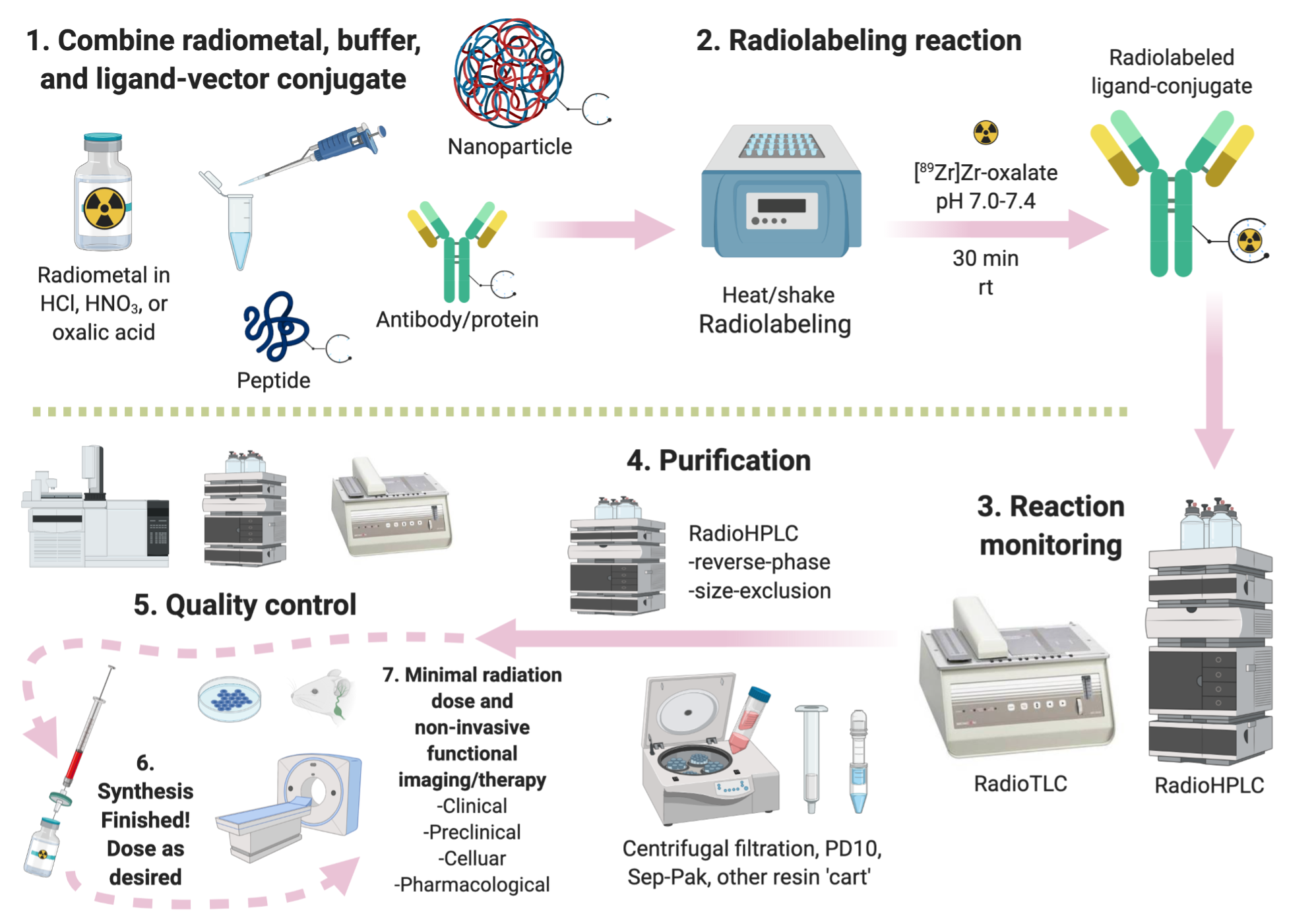 Figure 2. Graphical depiction of the radiopharmaceutical synthesis and testing pipeline, including radiolabeling, purification, quality control, and in vitro/in vivo evaluation.
Figure 2. Graphical depiction of the radiopharmaceutical synthesis and testing pipeline, including radiolabeling, purification, quality control, and in vitro/in vivo evaluation.
What can radiopharmaceuticals be used for?
Already well established in areas such as oncology,4 cardiology,5 neurology,6 and drug discovery,7 positron emission tomography (PET) is a nuclear imaging modality that has dramatically impacted drug development and biomedical research with an increasing number of novel probes being developed to target biomarkers associated with various diseases. PET has emerged as one of the most powerful molecular imaging technique available because it can minimally-invasively monitor biochemical reactions inside living people (i.e. no biopsy). The body is effectively “transparent” to nuclear imaging techniques due to the high tissue penetration of gamma rays. As such, PET is a valuable research tool used to evaluate disease promoting biochemical processes, evaluate response to drugs, and diagnose diseases such as cancer, heart-conditions, and Alzheimer’s. From ~2014-2020 USask and the Sylvia Fedoruk Canadian Centre for Nuclear Innovation has built and equipped a state-of-the-art cyclotron facility dedicated to the discovery of new compounds as potential nuclear imaging and therapy drugs.
Further, radiolabeling a new drug or interesting molecule aids drug development by providing an unparallel ability to study pharmacokinetics using quantitative PET imaging and very precise and easy ex vivo radioactivity distribution assays. PET is an increasingly common imaging tool that can uniquely provide functional imaging with quantitative data. PET enables the study of in vivo biochemistry, non-invasive receptor-occupancy reporting, 4D imaging (3D and longitudinal over-time measurements), drug efficacy studies, and quantitative PET signal for in vivo radiotracer concentration measurements over time (pharmacokinetics). Simply put, you can watch where the radiolabeled-pharmaceutical goes in the body and at what speed, how long it takes in transit (how long in the blood?), how long it stays at each stop (tissues/organs), which tissues it is retained in, and how it is excreted. With different radiopharmaceutical drugs, diseases such as Parkinson’s, Alzheimer’s, many other brain disorders, infectious fungal/bacterial/viral diseases, and any cancer type can be targeted.
Are radiopharmaceuticals clinically and commercially viable?
Minimally invasive nuclear imaging modalities such as PET are currently the only way to truly apply the concepts of personalized medicine in the clinic. The combined power of the functional molecular imaging of PET and the unprecedented efficacy of targeted radionuclide therapy has secured the role of radiopharmaceuticals as “theranostics/radiotheranostics” in the future of cancer care.8-10 Although nuclear imaging has been used for many decades and even the concept of radiotheranostics has been known for over 70 years, truly next-generation radiotheranostics have seen a surge in interest over the last decade.8 Radiotheranostic sales only started to surge in 2013 with the approval of the radiotherapeutic Xofigo (radium-223 dichloride), where total revenue from other radiopharmaceuticals was ~$4 billion USD.8 By 2020, global sales of radiotheranostics (mostly just Xofigo) reached ~$1.5 billion, with total radiopharmaceutical revenue reaching ~$6-7 billion USD.8 By 2025 a majority of the growth in radiopharmaceutical sales is predicted to be radiotheranostics with total radiopharmaceutical revenue approaching ~$10-14 billion.8
Recent examples of successful radiotheranostics are the prostate-cancer agent [177Lu]Lu-PSMA-617 which was owned by USA-based Endocyte and purchased by Novartis in 2018 for $2.1 billion, and the neuroendocrine targeting Lutathera [177Lu]Lu-DOTATATE owned by Advanced Accelerator Applications and sold to Novartis for $3.9 billion in 2018.9 Canada has a long history of radiopharmaceutical excellence, including seminal work on cobalt-60 radiotherapy by Sylvia Fedoruk and colleagues at the University of Saskatchewan in the 1950s. Europe, Canada, and many other places in the world are embracing radiopharmaceuticals as critical tools in personalized medicine, with cost being the major limiting factor.11, 12 In 2018 the radiopharmaceutical market in Canada was estimated at ~$646.2 million CAD and was projected to grow to ~$1.176 billion CAD by 2032, although these figures don’t fully account for the recent success and demand for radiotheranostics, including emerging and yet to be approved alpha-therapies such as actinium-225.13 A recent Canadian success story is Fusion Pharmaceuticals which emerged from McMaster and the Centre for Probe Development and Commercialization. Fusion is developing targeted radiotheranostics based on actinium-225 tethered to antibodies and peptides and as of ~2022 has raised over $125 million in Series B funding, receiving one of the largest single private investments in Canadian Biotech history. Another recent Canadian success story is Alpha-9 Theranostics, which emerged from the University of British Columbia, TRIUMF, and BC Cancer, and as of ~2022 has raised ~$85 million in Series B funding.
Impact of radiopharmaceutical research on Canada and Saskatchewan
Molecular imaging is revolutionizing our understanding of normal and pathological processes in plants, animals, and humans by providing tools for visualizing and quantifying physiological and biochemical events in real-time without invasive procedures (no cutting or physical probes inside the body). Given our aging population in North America and increasing global population, the burden that diseases such as cancer, Parkinson’s, viral infections, and drug-resistant bacterial infections have on individual families, world economies, and the national healthcare systems is rapidly increasing. In addition, the alarmingly swift emergence of multi-drug resistant bacteria and viral pandemics are now a global health risk that requires immediate action. Creating new radiopharmaceuticals and molecular imaging probes will assist in answering important questions related to human and animal health as well as both treating and diagnosing important diseases.
As radiopharmaceutical agents are delivered in micro-doses (nano-mole to pico-mole quantities), they are often classified as being “sub-pharmacological” and therefore have expedited testing guidelines from regulatory bodies such as the “Exploratory Investigational New Drugs (eIND)” program in the USA and the “Abbreviated New Drug Submissions” to Health Canada. This accelerates the progression of new radiopharmaceuticals through the development pipeline from the bench to the clinic (i.e. fewer preclinical studies needed), when compared to traditional pharmaceuticals. Commercialization and technology transfer of new radioactive drugs and chemical tools is a major goal of the Price Group.
Translation of new molecular imaging or radionuclide therapy drugs into the human or veterinary clinics and into Saskatchewan and the Canadian healthcare system is an ideal opportunity to impact both human and animal patient care. Successful research that leads to new radioactive drug products and/or patented chemical tools, startup companies, and industrial collaborations/contracts would create revenue and jobs for Saskatchewan and Canada while also disseminating new knowledge to the scientific and medical community. The next-generation radiopharmaceuticals being developed and studied in the Price Group should reduce radiation dose and unwanted side effects while simultaneously increasing the effectiveness of treatments for cancers and other diseases, thus improving public health. The intellectual property generated from these activities could lead to economic gains for USask (owner of research patents), Saskatchewan, and Canada in the form of licensing income, tax revenue, attraction of new investments, and job creation.
What role does the Price Research Group aim to play in the rapidly growing field of radiopharmaceutical development?
The Price Research Group combines existing molecules and reagents with our own novel chemical tools to solve real-world problems plaguing molecular imaging and radionuclide therapy of diseases such as cancer. Through a deep understanding of the chemistry used to construct these drugs and using clever solutions at the interface of chemistry and biology, we can carefully tune the properties of chemical and biological “smart drug” radiopharmaceuticals to maximize effectiveness and minimize side effects. One of the main goals of the Price Group is to improve efficacy and minimize side effects from nuclear imaging and targeted radionuclide therapy of diseases such as cancer.
If successful, the new radiopharmaceuticals and reagents/tools created from Prof. Price’s research program will improve early detection of a variety of cancers using molecular imaging techniques such as positron emission tomography (PET) imaging. This work includes radiotheranostic “smart drugs” that harness therapeutic radiometals and/or potent anticancer drugs. Radiolabeled antibodies are an example of a “smart drug” scaffold, as they can be created to target cancer cells with exquisite selectivity, while ignoring healthy tissues. Once injected, these “smart drugs” can selectively seek out cancerous cells by binding selectively to diseased cells that present specific receptors or biological processes. With cancer as an example, traditional chemotherapies tend to be toxic to all tissues and cause significant side effects, where targeted radionuclide therapy using antibodies or peptides as “smart drug” carriers have much higher selectivity. This results in greater cancer cell killing with lower toxicity and damage to healthy tissues. A majority of new radiopharmaceuticals use this “smart drug” principle.
In an interdisciplinary fashion, we study the wealth of scientific literature available about peptide and antibody drugs, including clinically tested and regulatory-body approved ones (e.g., trastuzumab/[89Zr]Zr-trastuzumab, [68Ga]Ga-NETSPOTä). We observe the positive and negative attributes of these drugs and then attempt to improve them from a first principles approach. For peptide-based drugs, we can take a successful peptide sequence (e.g. DOTA-TATE, NETSPOT) and re-synthesize it in-house using solid-phase peptide synthesis and then make a series of new and novel derivatives. We can also collaborate with companies or other research labs who create antibody drugs, which we can modify with our new chemical tools. Antibodies and peptides are perhaps the most successful drug-scaffolds for creating new radiopharmaceuticals due to their clinical abundance and success, their rapid development, and their versatile protein chemistry for conjugation reactions.14, 15 The Price Research Group chemically modifies and adapts these protein-based drugs into new hybrid molecules, which transforms a regular peptide or antibody into a radiopharmaceutical. To achieve this, we must perform precise chemical modifications of the selected antibodies and peptides and incorporate chemical tools such as radiometal-binding chelators and unnatural amino acids.
What are these new “chemical tools” and how are they incorporated into new radiopharmaceuticals?
The Price Group develops custom organic/radiochemical synthesis and purification procedures to make new chemical tools and radiopharmaceuticals. Their relatively high molecular weight makes common organic synthetic techniques a little more challenging, and overall polarity makes them hard to purify without significant experience. We design these molecules with molecular precision in both their chemical structures and their intended biological activity, and we must test them under real world conditions. After creating and validating a novel reagent/tool, we can conjugate them to antibody- and peptide-based drugs such as trastuzumab/nimotuzumab (antibodies, mAb drug) or octreotate/PSMA (peptides). For example, these custom reagents include chelators which can selectively bind different types of radioactive metal ions with extreme inertness and stability, fluorescent dyes to track and trace drugs or metabolism, enzyme/pH or otherwise activatable chemical triggers to selectively release a drug or radionuclide into tumour cells, or custom linkers to modify biological distribution. These custom molecules are highly specialized and we broadly call them reagents or “chemical tools”. These chemical tools are complicated to make and to study, and it typically take several years to develop each one.
These chemical tools must be “bifunctional”, meaning they must first do their primary job such as metal ion chelation or fluorescence, but they secondarily and critically must have a chemical moiety that can react quickly and stably to form a covalent and ideally irreversible linkage with a disease-targeting “smart drug” molecule such as a peptide or antibody (Figure 3). These are called bioconjugation reagents or bioconjugation moieties, which are essentially highly specialized chemical functional groups that are tuned to react under controlled aqueous conditions in a predictable way with some of the amino acids that antibodies and peptides are made from. The rich chemistry of amino acids ¾ the building blocks of peptides and antibodies ¾ enables chemical modification of peptides and antibodies with a variety of chemical tools.
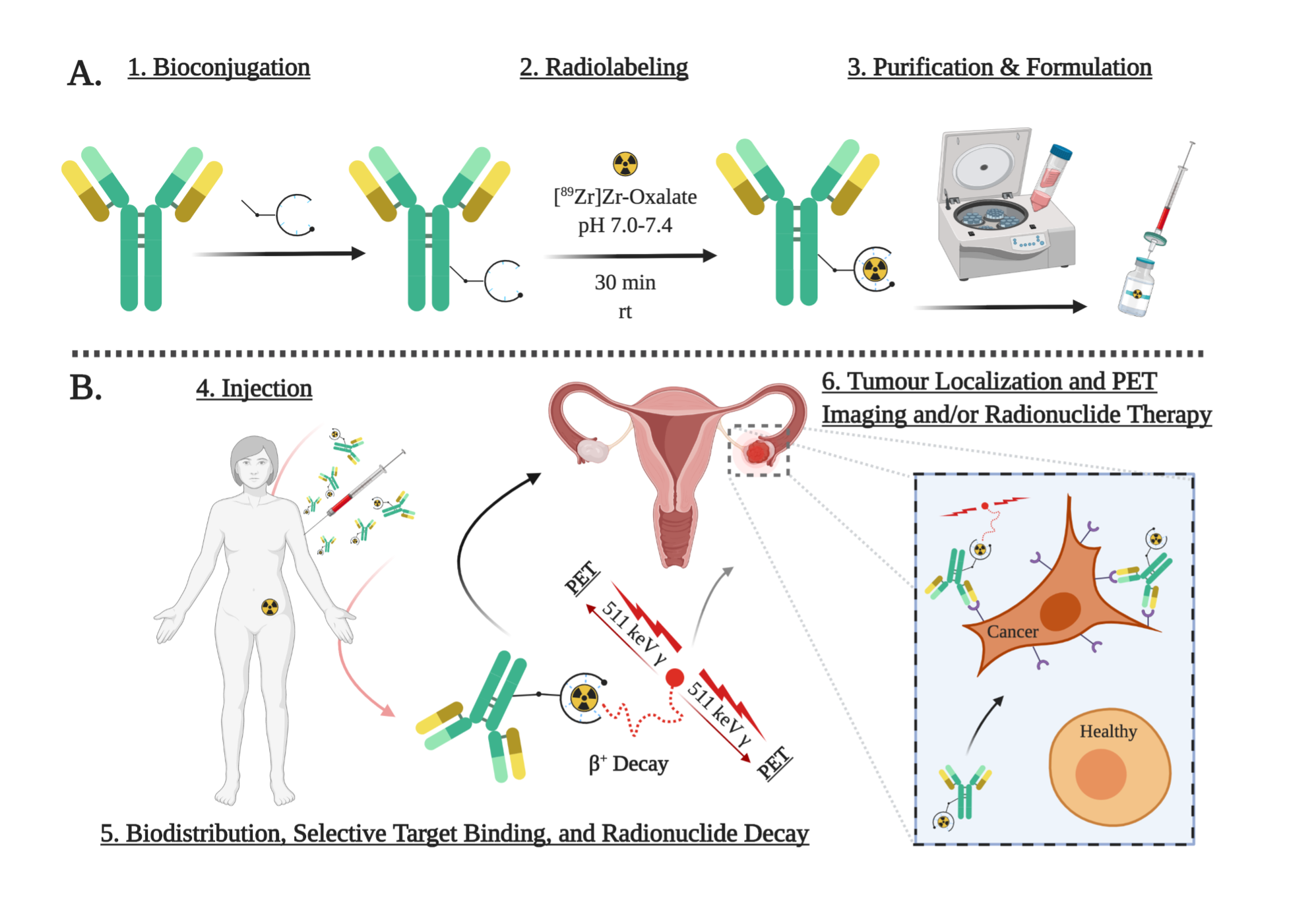
Figure 3. Graphical depiction of the process to transform a regular antibody into a radioimmunoconjugate (“smart drug” radiopharmaceutical based on antibodies), showing the radiolabeled antibody selectively binding to ovarian cancer cells while ignoring healthy cells. Figure created partially using biorender.com.
For example, after conjugating a bifunctional chelator to an antibody and radiolabeling it, the resulting radiometal-immunoconjugate is a hybrid molecule that has been transformed into a radiopharmaceutical with a new and powerful function. With stable attachment of a radiometal ion, the antibody is now a nuclear imaging or radionuclide therapy drug. In the same manner, we can conjugate fluorescent dyes for optical/fluorescence/microscopy imaging, and a variety of drugs such as antibody drug conjugates (ADCs). These provide cell-selective targeting for imaging and treating a variety of cancers and diseases. Although we mostly operate at the chemistry end of these efforts, we also perform radiochemistry and basic in vitro and in vivo work ourselves at USask. Through this pipeline we create new chemical tools but also new radiopharmaceuticals as proof-of-concepts for our tools.
Summary of the Price Research Group core projects
Prof. Price’s research program aims to create modular chemical tools which are designed and tested to improve existing radiopharmaceutical, peptide, and antibody-based drugs, and to be used as building blocks in the creation of next-generation radiopharmaceuticals. These chemical tools include: 1) radiometal-binding chelators for theranostic (dual therapy/nuclear imaging) applications, 2) near-infrared dyes with radioactive fluorine labeling capabilities, 3) functional linkers to improve pharmacokinetic and therapeutic properties of radiometal-based drugs, 4) novel bioconjugation reagents to improve conjugation yields and stability for attaching chemical payloads (e.g. radiometal, chemotherapy drug) to antibodies and proteins, and 5) enzyme-cleavable linkers for enhanced tumor delivery of a variety of chemical payloads from vector-conjugates such as antibody-drug conjugates and peptide-conjugates. Visit the publications section of the Group website to see peer-reviewed examples of this research.
Although there are many ongoing projects and collaborations the Group has two main focuses. Core projects in the Group involve the design and synthesis of new bifunctional chelators for a variety of radiometals, and the design and synthesis of novel linkers and bioconjugation strategies. These linkers include enzyme-cleavable linkers for antibody drug conjugates and radiopharmaceuticals, and linkers bearing permanent zwitterion moieties and/or enzyme-cleavable linkers for enhanced polarity and solubility with decreased uptake in healthy tissues and the kidneys. These chelators and linkers are chemically modular and much like LEGO they can be connected to many different molecules to create a theoretically limitless number of new radiopharmaceutical drugs.
Chelators for radiometals: Several pressing issues in this field revolve around the challenging chemistry required to make suitable chelators and linkers that can be attached to drug vectors (e.g. peptides, antibodies) without negatively altering their properties (e.g. reduced water solubility, increased “sticking” in excretory organs). The chelator and the linker can cause drastic changes to the biological distribution and drug activity of the final radiopharmaceutical. Chelators must tightly hold radiometals against all competition for binding in vivo (e.g., abundant blood proteins such as transferrin and albumin).16, 17 The radiometal is effectively chemically tethered to a drug using a chelator, and if it is released from the grasp of the chelator in vivo, the “free” radiometal will be partially excreted (e.g., urine, feces) and partly taken up by other tissues, such as the bones, liver, and kidneys, causing unwanted radiation exposure to a patient’s body (Figure 4).3, 16, 17 The major hurdles in the study of new chelators is their challenging chemical synthesis, protection chemistry (abundance of polar and charged functional groups), and purification. If new chelators are created that can bind radiometal ions with greater strength and inertness, the amount of unwanted radiation exposure that patients are exposed to can be decreased. Chelators that can bind unique combinations of different radiometals are also of strong interest.
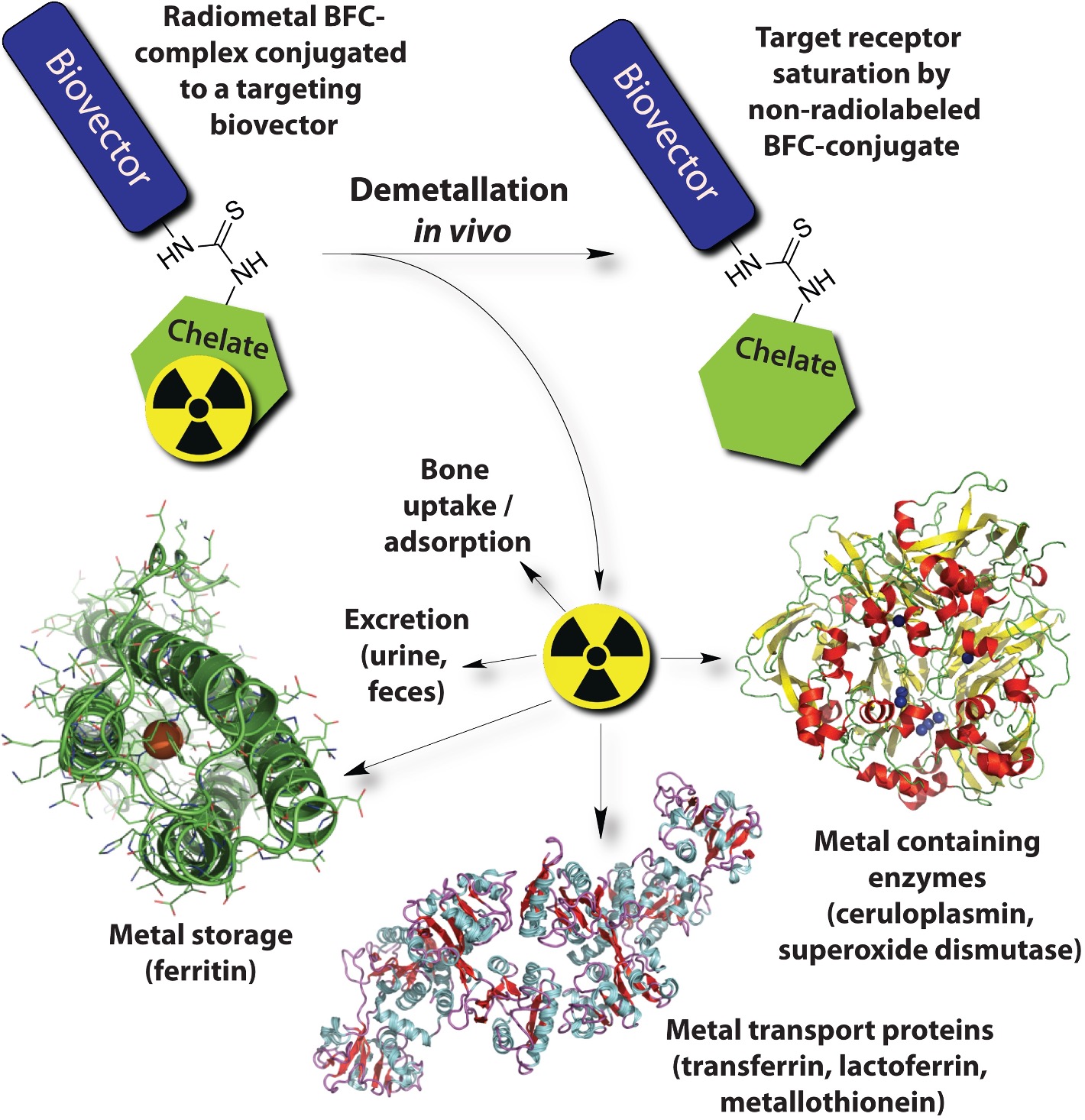
Figure 4. Cartoon illustrating some of the possible biological fates and consequences of radio-demetallation (radiometal being released from the grasp of the chelator) in vivo (solid-state structures of ferritin H-chain homopolymer PDB file 1FHA, ceruloplasmin PDB file 2J5W, and apo-transferrin PDB file 2HAV shown). Figure from textbook article.3
Peptide-based radiopharmaceuticals: Peptides are a very popular class of targeting vectors used for creating radiopharmaceuticals, because high affinity peptides can be produced quickly to many different targets and they can be modified quickly. Peptides can be generated to theoretically any biological target, including sub-types of cancers and other diseases. Most molecular imaging probes, such as peptides, have a large amount of non-specific binding and retention in non-target tissues (e.g. muscle, fat, skin, kidneys, other excretory organs). The quantity of radiometallated peptide that sticks or passes through the kidneys is often a majority of the dose of radiopharmaceutical, and the kidneys are often the dose-limiting organ. The dose-limiting organ effectively sets a limit on the quantity of radiopharmaceutical you can administer while safely protecting the dose-limiting organ, which means it also limits the amount of radioactivity that can be delivered to tumours.
In an attempt to reduce the tendency of radiometallated peptides to be taken up into and retained in the kidneys, we are exploring new linkers with unique charge distribution. We have evaluated our first generation of new linkers using the blockbuster radiometal-based radiopharmaceutical peptide DOTA-TATE (NETSPOT/LUTATHERA) as a scaffold. The Zwitterionic nature of these linkers has the effect of substantially increasing the polarity/solubility and the speed of kidney clearance, while also reducing uptake and retention of radiometals in the kidneys. Modular linker groups with a variety of charges, functional groups, and spacers are being designed, synthesized, and investigated in the Group for a simple and efficient way to tune the polarity and biodistribution of any small drug/peptide based radiopharmaceutical. An additional class of novel linker groups being studied in the Group contain functional groups that aim to improve tumour retention. Our first-generation chemical tools are being tested and validated with derivatives of the blockbuster peptide-based radiopharmaceutical NETSPOTä/LUTATHERAä (DOTA-TATE).
Collaborative research
The Price Research Group is takes a highly collaborative and interdisciplinary approach to research. We are working with Prof. Ron Geyer (USask Faculty of Medicine, Pathology and Laboratory Medicine) to conjugate several of our best chelators to a clinically used antibody “nimotuzumab”. Profs. Geyer and Humphrey Fonge have previously worked with collaborators in Cuba to create [89Zr]Zr-nimotuzumab and have completed pre-clinical studies for imaging triple negative breast cancer, and now a clinical trial is ongoing at USask/RUH for PET imaging in human patients. The new chelator created by the Group has recently been conjugated to nimotuzumab and we are currently investigating performance with several radionuclides.
We are also working on testing new bioconjugation chemistries and chelator synthesis with Prof. Humphrey Fonge (Medical Imaging, USask Medicine) for antibody drug conjugates. We also have a unique collaboration with a surgeon at Royal University Hospital Dr. Mike Moser who is a specialist in cancer and a NanoKnife technique, for which we are investing some new strategies along with Prof. Phenix. The Price Group also has collaborations with Prof. Chris Phenix (Chemisty, USask) for creating next-generation antibody drug conjugates (ADCs), Profs. Graham George and Ingrid Pickering (Geological Sciences, USask) for studying metal-chelate complexes using Synchrotron EXAFS spectroscopy and computational modeling (DFT), Prof. Behzad Toosi (Veterinary Department, USask) for studying new radiopharmaceuticals in animal diseases, Prof. Ildiko Badea (Pharmacy and Nutrition, USask) for creating new radiolabeled nanodiamond nanoparticles, Prof. Brian Zeglis (Hunter College, NYC, USA) for new thiol-selective bioconjugation reagents, Prof. Valery Radchenko (TRIUMF, UBC, Vancouver, Canada) for testing new chelators with therapeutic radionuclides, and Prof. James Inkser (McMaster April 2023, Hamilton, Canada) and several others.
What research experiences will members of the Price Research Group have?
Core experiences that trainees in the Price Group will have could include challenging and lengthy custom-organic syntheses of specially designed chelators, fluorescent dyes, enzyme-activatable linkers, unnatural amino acids, and bioconjugation reagents. We are a chemistry-based group with applications in medicine. We heavily rely on normal- and reverse-phase chromatography for purifications, including semi-automated and high-pressure systems with in-line UV/Vis/radiation detectors and semi-automated fraction collectors. This process is not routine or standardized, as many molecules we synthesis are new, and each new molecule contains its own unique chemical/biochemical puzzle to be solved. Because most of the molecules/chemical tools we synthesize are in-house designed and are new molecules that have rarely or never been synthesized before, this requires substantial time, effort, and skill. Analogous to playing with atomic-sized LEGO building blocks, to obtain the desired molecules using multi-step chemical synthesis we must first create a synthetic roadmap that must utilize commercially available chemical precursors (building blocks). As such, issues with supply chains, commercial availability, or prices can affect our efforts and alter our timelines. The process of synthesizing these large (400-2000 g·mol-1) and complicated molecules is turbulent and challenging, particularly because they usually contain many highly polarized and/or acidic chemical moieties that can often participate in hydrogen bonding (intra- and inter-molecular).
Opportunities in radiochemistry and the Price Research Group
Both organic synthesis and radiochemistry are technical and challenging fields of science, and each have long and steep training curves. As such, the Price Group mostly recruits Ph.D. students and not M.Sc. There is a general world-wide shortage of people trained in radiochemistry and therefore a great demand for both trainees and employees with combinations of chemistry/radiochemistry/radiopharmacy training and expertise. Although vacancies in the Price Group are always limited due to academic lab space and funding limitations, the Price Group is typically comprised of ~5-10 enthusiastic people including undergraduate and graduate students, postdoctoral fellows, and research associates. They all work together in a team-minded environment to perform the research described above and are the lifeblood of the Price Group. Undergraduate students in the Price Group can gain experience or exposure to multi-step organic synthesis, purification, and characterization of a variety of molecules. These molecules are made with the goal of being evaluated by radiochemistry and in vitro/in vivo validation.
The lab and research program are both run and supervised by Dr. Eric W. Price, with support from the University of Saskatchewan, the College of Arts and Science, the Department of Chemistry, and many peer-reviewed funding organizations. The Saskatchewan Centre for Cyclotron Sciences is an on-campus radiochemistry user facility with world-class facilities that is operated by the Sylvia Fedoruk Canadian Centre for Nuclear Innovation, which has provided generous funding to the Group and is critical for radiochemical research at USask.
Other research groups on campus that perform research using radiochemistry include but are not limited to Profs Phenix, Geyer, Fonge, Dadachova, Krol, Badea, and Sicilliano.
For more detailed information about Prof. Price’s research program at USask, visit https://research-groups.usask.ca/price/index.php or contact by email at eric.price@usask.ca.
Professor Eric W. Price, Ph.D.
References
(1) Ballinger, J. R. Theranostic radiopharmaceuticals: established agents in current use. The British Journal of Radiology 2018, 91 (1091), 20170969. DOI: 10.1259/bjr.20170969.
(2) Weber, W. A.; Czernin, J.; Anderson, C. J.; Badawi, R. D.; Barthel, H.; Bengel, F.; Bodei, L.; Buvat, I.; DiCarli, M.; Graham, M. M.; Grimm, J.; Herrmann, K.; Kostakoglu, L.; Lewis, J. S.; Mankoff, D. A.; Peterson, T. E.; Schelbert, H.; Schöder, H.; Siegel, B. A.; Strauss, H. W. The Future of Nuclear Medicine, Molecular Imaging, and Theranostics. J. Nucl. Med. 2020, 61 (Supplement 2), 263S. DOI: 10.2967/jnumed.120.254532.
(3) Price, E. W.; Orvig, C. The Chemistry of Inorganic Nuclides (86Y, 68Ga, 64Cu, 89Zr, 124I). In The Chemistry of Molecular Imaging, John Wiley & Sons, Inc, 2014; pp 105-135.
(4) Beadsmoore, C.; Newman, D.; MacIver, D.; Pawaroo, D. Positron Emission Tomography Computed Tomography: A Guide for the General Radiologist. Canadian Association of Radiologists Journal 2015, 66 (4), 332-347. DOI: http://dx.doi.org/10.1016/j.carj.2015.02.003.
(5) Sdringola, S.; Johnson, N.; Gould, K. L. Clinical Cardiac Positron Emission Tomography. In Coronary Artery Disease, Willerson, J. T., Holmes, J. D. R. Eds.; Cardiovascular Medicine, Springer London, 2015; pp 263-281.
(6) McConathy, J.; Sheline, Y. I. Imaging Biomarkers Associated With Cognitive Decline: A Review. Biological Psychiatry 2015, 77 (8), 685-692. DOI: http://dx.doi.org/10.1016/j.biopsych.2014.08.024.
(7) Mammatas, L.; Verheul, H. W.; Hendrikse, N. H.; Yaqub, M.; Lammertsma, A.; Menke-van der Houven van Oordt, C. W. Molecular imaging of targeted therapies with positron emission tomography: the visualization of personalized cancer care. Cell Oncol. 2015, 38 (1), 49-64. DOI: 10.1007/s13402-014-0194-4.
(8) Herrmann, K.; Schwaiger, M.; Lewis, J. S.; Solomon, S. B.; McNeil, B. J.; Baumann, M.; Gambhir, S. S.; Hricak, H.; Weissleder, R. Radiotheranostics: a roadmap for future development. The Lancet Oncology 2020, 21 (3), e146-e156. DOI: https://doi.org/10.1016/S1470-2045(19)30821-6.
(9) Cutler, C. S. Economics of New Molecular Targeted Personalized Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49 (5), 450-457. DOI: https://doi.org/10.1053/j.semnuclmed.2019.07.002.
(10) Group, T. A. T. W. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncology 2018, 4 (12), 1765-1772. DOI: 10.1001/jamaoncol.2018.4044 (acccessed 11/20/2020).
(11) Decristoforo, C.; Neels, O.; Patt, M. Emerging Radionuclides in a Regulatory Framework for Medicinal Products – How Do They Fit? Frontiers in Medicine 2021, 8, Mini Review. DOI: 10.3389/fmed.2021.678452.
(12) World Nuclear Association: Radioisotopes in Medicine. 2021. https://world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx (accessed 2022 April 7 2022).
(13) adMare Bioinnovations, Centre for Probe Development and Commercialization: Discover the Medical Isotope & Radiopharmaceutical Landscape in Canada. 2021. https://www.admarebio.com/discover-the-medical-isotope-radiopharmaceutical-landscape-in-canada/ (accessed 2022 April 7 2022).
(14) Dammes, N.; Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020, 10 (2), 938-955, Review. DOI: 10.7150/thno.37443.
(15) Minczeles, N. S.; Hofland, J.; de Herder, W. W.; Brabander, T. Strategies Towards Improving Clinical Outcomes of Peptide Receptor Radionuclide Therapy. Curr. Oncol. Rep. 2021, 23 (4), 46. DOI: 10.1007/s11912-021-01037-7.
(16) Price, E. W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43 (1), 260-290. DOI: DOI: 10.1039/c3cs60304k.
(17) Wadas, T. J.; Wong, E. H.; Weisman, G. R.; Anderson, C. J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. (Washington, DC, U. S.) 2010, 110 (5), 2858-2902. DOI: 10.1021/cr900325h.
