Paper published in J. Phys. Chem. A
Excimer-Suppressed and Oxygen-Tolerant Photophysics of “Arm-like” Substituted Pyrene Derivatives
Wenlong Li, Stephen Awuku, Jenna N. Merk, Marc R. MacKinnon, and Amy L. Stevens.
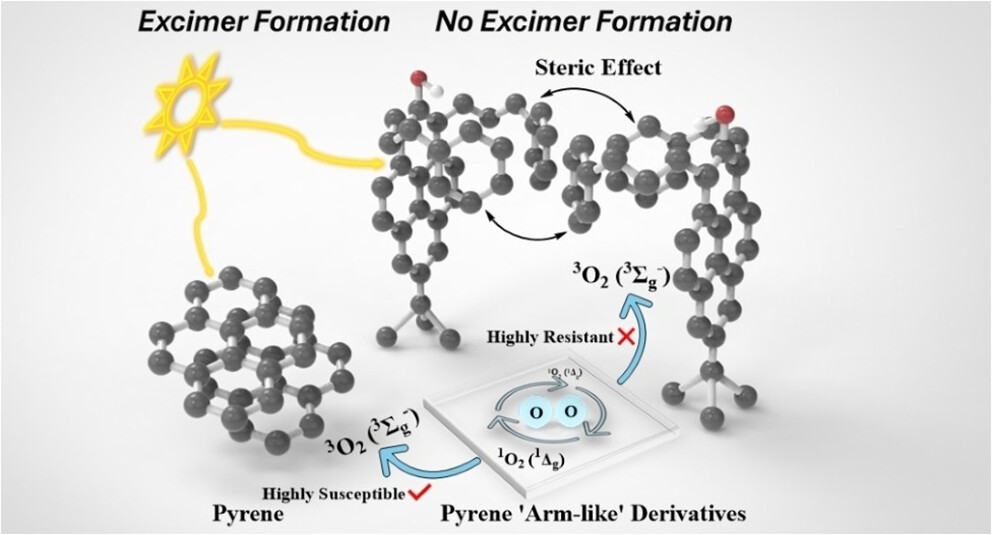
Pyrene-functionalized materials are extensively employed in photoluminescent applications, owing to their extended π-conjugation and favorable photophysical properties. However, their luminescent performance is often attenuated by π–π stacking-driven excimer formation and molecular oxygen quenching. To mitigate these undesirable effects, a novel class of 7-tert-butylpyren-2-ol derivatives with extended “arm-like” substituents at the 1,3-positions have been synthesized and their luminescent properties in solution have been thoroughly investigated. While the 2- and 7-positions of the pyrene core are frequently modified with hydroxyl and tert-butyl groups, this work presents the first introduction of “arm-like” substituents at the 1,3-positions. The stretched-out “arm-like” substituents not only introduce steric bulk to suppress excimer formation but also change the symmetry class of pyrene and modulate electron density at its 1,2,3,7-positions. These effects tune pyrene’s energy levels and result in moderate (0.4) to high (0.7) fluorescence quantum yields and shorter-lived fluorescence lifetimes ranging from ca. 20 to 40 ns. These shorter lifetimes lead to a reduction of the pyrene derivatives’ susceptibility to energy scavenging by molecular oxygen. In addition, the specific form of the “arms” are important. Alkyl-containing arms and alkenyl-containing arms exhibit different decay pathways, which is reflected by their disparate nonradiative rates. Thus, the introduction of “arm-like” modifications represents a promising approach to modulate the photophysical behavior of an important class of polycyclic aromatic hydrocarbons, highlighting their applicability in next-generation electronic and optoelectronic systems.

