Paper published in Langmuir
Immiscible anionic gemini surfactant-perfluorinated fatty acid Langmuir monolayer films
Jeveria Rehman, David Sowah-Kuma, Amy L. Stevens, Wei Bu, Matthew F. Paige
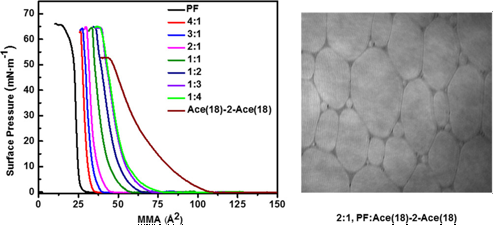
A new member of the N,N,N′,N′-dialkyl-N,N′-diacetate ethylenediamine family of anionic gemini surfactants has been synthesized, and its miscibility with the model perfluorocarbon, perfluorotetradecanoic acid (PF), has been investigated in monolayer films at the air–water interface. Thermodynamics of mixing and the accompanying changes in the mixed film structure have been probed using a combination of compression isotherm measurements supported by Brewster angle microscope imaging and X-ray scattering measurements, and results have been compared with those collected for a previously studied, shorter tail chain variant of the surfactant. Thermodynamic measurements showed that the gemini surfactant and perfluorotetradecanoic acid were immiscible, with weak repulsive interactions, manifesting as small positive deviations from ideal mixing, observed between the two film components. Films were highly textured, with micrometer-scale, phase-separated domains readily detectable. Grazing incidence X-ray diffraction measurements showed that the gemini surfactant was disordered in the monolayers, whereas the perfluorocarbon formed discrete crystallites in the disordered matrix. Despite the small deviations from ideal mixing detected in the thermodynamic measurements, the X-ray measurements indicated that the presence of the gemini perturbs the PF crystal lattice from that of pure PF. Finally, X-ray reflectivity measurements showed that the addition of equimolar PF to the gemini monolayer induces a significant increase in the nominal head group thickness of the film, suggesting that interactions between the two surfactants can lead to structural rearrangements of gemini’s head group near to the water surface.
https://doi.org/10.1021/acs.langmuir.9b01554

