Background
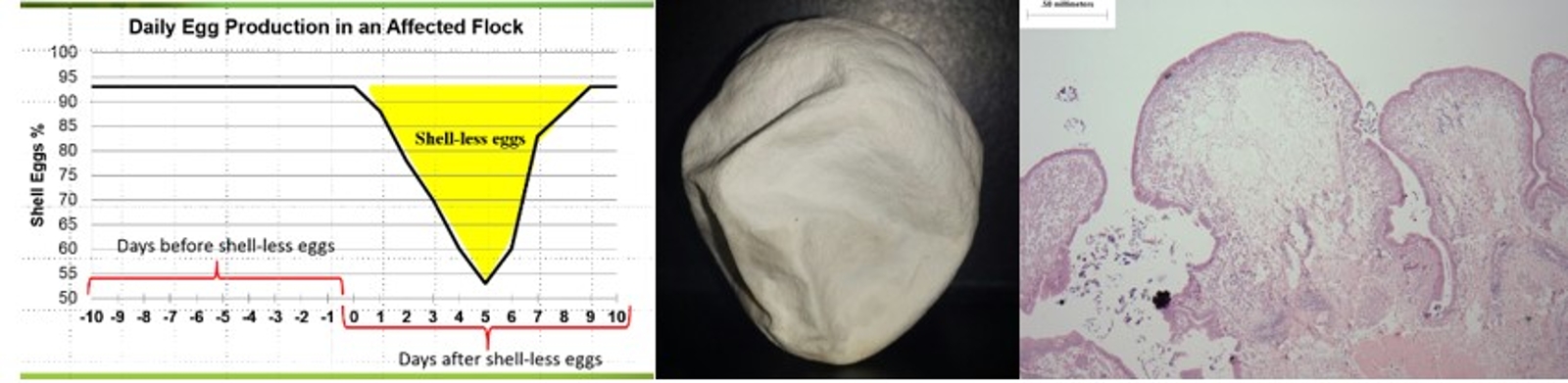
Apparently healthy layer flocks with no clinical signs of disease with an acute increase in shell-less eggs of 30-40% for 3-4 days has been reported in Manitoba, Saskatchewan and Alberta. Shell quality returned to normal after 7-10 days. Histopathology of the uterus showed the presence of edema and inflammatory cells suggesting a viral infection. The swelling of the uterus thus resulted in poor calcium deposition on the egg.
Shell-less eggs may be caused by low pathogenic avian influenza, duck adenovirus-1 (egg drop syndrome), Newcastle disease virus (NDV), avian encephalomyelitis (AEV) or infectious bronchitis virus (IBV). The causative agent until this point had not been previously diagnosed.
We hypothesized that shell-less egg syndrome (SES) was associated with variant IBV. IBV replicates in both young and egg producing chickens. It is primarily considered a respiratory virus, but lesions caused by IBV can be found in the respiratory and reproductive tracts. Some IBV strains can also affect the urinary system. Therefore, mortality due to IBV infections can be seen in young chickens or reduction of egg quality and production in adult birds.
In order to control IBV, vaccination with live attenuated viral vaccines are commonly used. Vaccine IBV strains include Massachusetts, Connecticut, Arkansas and combinations thereof. However, there has been an emergence of new variant IBV strains leading to infectious bronchitis outbreaks in vaccinated flocks. Strains used in vaccines may also spread amongst individual birds in a flock regaining their virulence or disease causing capacity. It has been shown that vaccines against IBV should be developed to understand the locally prevalent strains in order to have protective responses.
Our Research
Serological monitoring of IBV, Newcastle disease virus (NDV) and avian encephalomyelitis (AEV) in Saskatchewan and Alberta. Tissues from 3-5 cull birds (trachea, lung, oviduct, kidney and cecal tonsil) when available for surveillance samples and serological and tissue sampling from cases of shell-less egg syndrome.
Vaccination deficiencies detected. Antibody levels are lower than expected. Over 80% of the flocks examined were positive for IBV. 100% of SES cases were positive for IBV. Of these cases 67% were positive for the IBV genome. These results indicate layer flocks with acute SES or a history of SES had been infected or exposed to IBV.
Virus was recovered from tissue (trachea, lung, oviduct, kidney and cecal tonsil) collected from routine samples and cases of SES and was used to infect specific pathogen free (SPF) layers. SPF laying hens were administered the isolates by the intra-tracheal route. Post-infection with the field isolate, shell-less eggs and irregular egg production were recovered from the infected group. Virus was recovered from swab and tissue samples at the end of the trial. There was serosal and mucosal congestion and microscopic edema in the oviduct of affected birds. SES was reproduced experimentally using field isolates. These isolates were determined to be IBV.
This research was conducted in collaboration with Dr. Faizal Careem, Faculty of Veterinary Medicine, Univeristy of Calgary.


